by Jason Wasserman MD PhD FRCPC
July 3, 2025
Chronic lymphocytic thyroiditis, also known as Hashimoto’s thyroiditis, is a common autoimmune condition that affects the thyroid gland. The thyroid is a small, butterfly-shaped gland located in the front of the neck that produces hormones responsible for regulating metabolism, energy levels, and numerous other vital body functions.
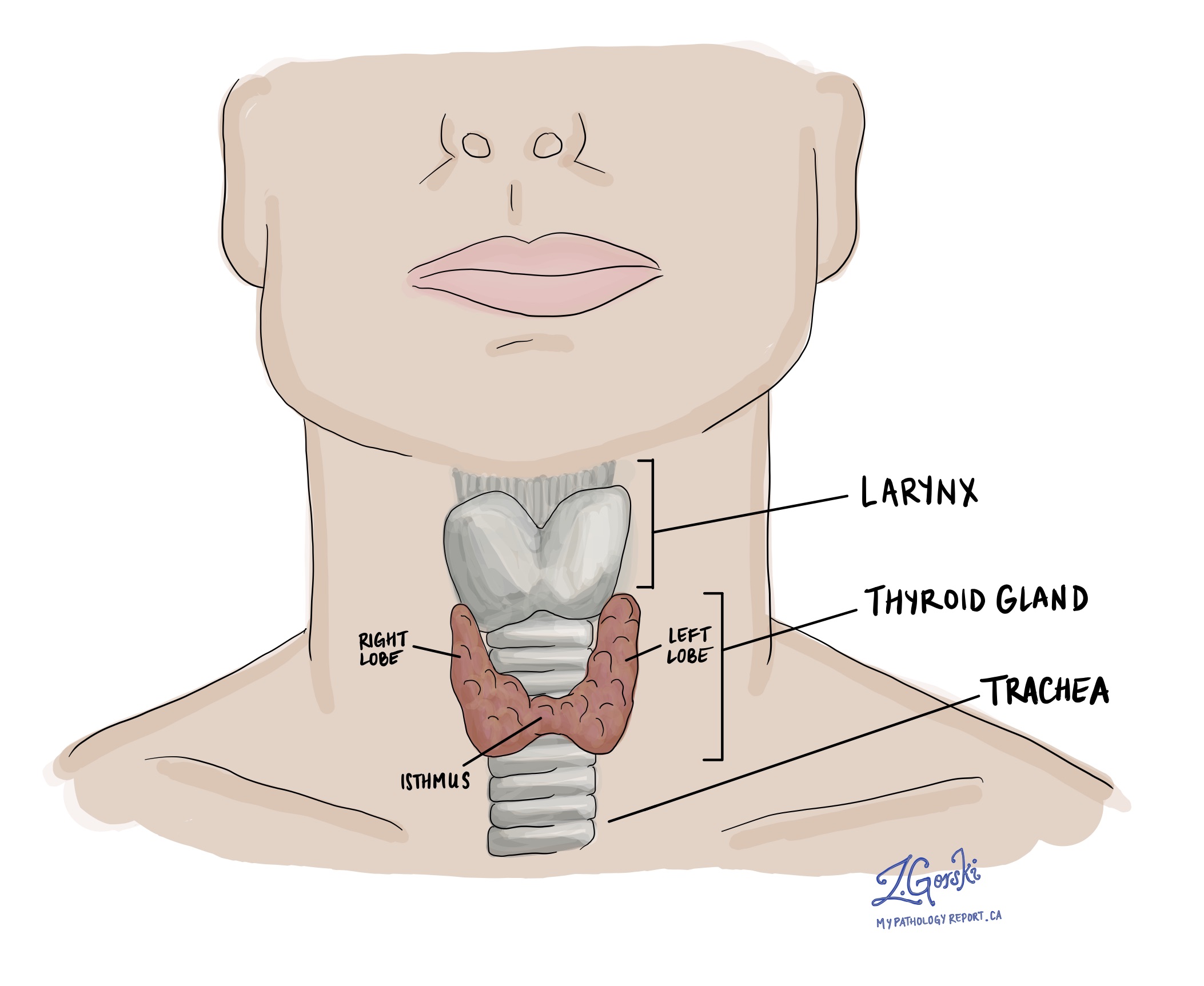
In this condition, the immune system mistakenly attacks the thyroid gland, resulting in long-term inflammation and gradual damage to the thyroid cells. Over time, the thyroid becomes less able to produce enough hormones, leading to a condition called hypothyroidism (an underactive thyroid).
Chronic lymphocytic thyroiditis is one of the most common causes of hypothyroidism, especially in middle-aged women.
What are the symptoms of chronic lymphocytic thyroiditis?
Many people with chronic lymphocytic thyroiditis initially have no symptoms, and the condition may be discovered during routine blood work or imaging performed for another reason.
As the thyroid gland becomes more damaged and hormone production decreases, symptoms of hypothyroidism may develop. These may include:
-
Fatigue and low energy.
-
Weight gain.
-
Cold intolerance (feeling unusually cold).
-
Constipation.
-
Dry skin.
-
Depression or mood changes.
-
Slowed heart rate.
-
Menstrual irregularities.
-
Muscle weakness or cramps.
-
Puffy face or swelling in the neck (goiter)
Symptoms tend to develop slowly and may be mild at first, making them easy to overlook.
What causes chronic lymphocytic thyroiditis?
Chronic lymphocytic thyroiditis occurs when the immune system malfunctions. In a healthy body, the immune system defends against viruses and bacteria. But in autoimmune disorders like Hashimoto’s thyroiditis, the immune system mistakenly produces autoantibodies—special proteins that attack the body’s own tissues.
In this case, the autoantibodies target proteins on thyroid cells. This triggers inflammation and attracts more immune cells to the thyroid gland. Over time, the repeated immune attacks lead to tissue damage and a decline in thyroid hormone production.
The exact reason why the immune system behaves this way is not fully understood, but several risk factors have been identified:
-
Family history: People with a close relative who has an autoimmune disease (such as type 1 diabetes or lupus) are at higher risk.
-
Gender: Women are much more likely than men to develop this condition.
-
Age: It is most common in middle-aged adults, especially women over 60.
-
Other autoimmune disorders: Conditions such as rheumatoid arthritis, type 1 diabetes, or celiac disease increase the risk.
-
Radiation exposure: Exposure to radiation (from medical treatments or environmental sources) may increase the risk.
-
Genetic factors: Certain genes may increase the likelihood of developing autoimmune thyroid diseases in some individuals.
How is chronic lymphocytic thyroiditis diagnosed?
Diagnosis is usually made using a combination of blood tests and, in some cases, a tissue biopsy.
-
Blood tests: These are used to assess thyroid function and to detect autoimmune activity. Your doctor may order:
-
Thyroid-stimulating hormone (TSH): Usually high in hypothyroidism.
-
Free T4: Often low in hypothyroidism.
-
Thyroid peroxidase antibodies (TPOAb): These are commonly found in people with Hashimoto’s thyroiditis.
-
Thyroglobulin antibodies (TgAb): Another type of antibody is sometimes present.
-
-
Ultrasound: Imaging may show a diffusely enlarged thyroid with a coarse or heterogeneous texture.
-
Fine-needle aspiration biopsy (FNAB): Occasionally, a biopsy is performed if a nodule is present or the diagnosis is uncertain. A small sample of thyroid tissue is examined under the microscope by a pathologist.
What does chronic lymphocytic thyroiditis look like under the microscope?
When viewed under the microscope, the thyroid tissue shows signs of long-term immune system activity. Pathologists often see:
-
Lymphocytes and plasma cells: These are immune cells that infiltrate the thyroid tissue, causing inflammation. Their presence is the hallmark of chronic lymphocytic thyroiditis.
-
Lymphoid follicles: These are organized clusters of immune cells, usually seen in lymph nodes. Their presence in the thyroid indicates that the disease has been present for a long time.
-
Oncocytic (Hürthle cell) change: Some thyroid cells become enlarged and filled with pink-staining material. This is a common reactive change and does not indicate cancer.
-
Cytologic atypia: Some thyroid cells may look mildly abnormal, but these changes are usually due to inflammation rather than cancer.
These microscopic features help confirm the diagnosis and distinguish chronic lymphocytic thyroiditis from other thyroid conditions.
What are the next steps after diagnosis?
Once the diagnosis is confirmed, treatment depends on how well the thyroid is functioning:
-
If thyroid hormone levels are normal, your doctor may recommend regular monitoring without the need for medication.
-
If you develop hypothyroidism, your doctor may prescribe levothyroxine, a synthetic form of thyroid hormone, to replace what your body is no longer making.
-
If the thyroid becomes enlarged (a goiter) and causes symptoms such as trouble swallowing or breathing, surgery may be considered, although this is uncommon.
-
Individuals with this condition should undergo regular follow-ups to monitor thyroid function and adjust treatment as necessary.
Questions to ask your doctor
-
What do my thyroid hormone levels show?
-
Do I have antibodies that suggest autoimmune thyroid disease?
-
Will I need medication to replace my thyroid hormones?
-
How often should I have my thyroid function tested?
-
Are there lifestyle changes or supplements that may help?
-
Should I be tested for other autoimmune conditions?
-
Is there a chance my condition will improve over time?



