by Jason Wasserman MD PhD FRCPC
November 1, 2024
Oncocytic adrenocortical carcinoma is an adrenal gland cancer characterized by oncocytic cells. These abnormal cells have an excessive number of mitochondria, giving them a distinctive granular and eosinophilic (pink) appearance under the microscope.
Characteristics of oncocytic cells:
- Appearance: Large, with abundant granular cytoplasm.
- Origin: Derived from adrenal cortical cells.
- Function: May retain some hormonal activity but often present as non-functional.

Symptoms of oncocytic adrenocortical carcinoma
Oncocytic adrenal adrenocortical can present with various symptoms, which may be caused by the tumour’s mass effect (the tumour pressing on surrounding organs) or hormonal imbalances resulting from the tumour’s functional activity. Symptoms can be categorized into two main groups: those related to mass effect and those related to hormone production.
Symptoms related to mass effect
- Abdominal pain or discomfort: Due to the pressure exerted by the growing tumour.
- Palpable abdominal mass: Sometimes, the tumour can be large enough to be felt through the abdominal wall.
- Weight loss: Unexplained weight loss can occur as the tumour progresses.
- Early satiety: Feeling full after eating small amounts of food due to pressure on the stomach.
- Back pain: Pain radiating to the back if the tumour compresses nearby structures.
Symptoms related to hormone production
Oncocytic adrenal adrenocorticals can be functional (hormone-producing) or non-functional. Functional tumours can cause symptoms related to the excess production of adrenal hormones such as cortisol, aldosterone, and androgens.
Cushing’s syndrome (excess cortisol)
- Weight gain: Particularly in the face, neck, and abdomen.
- Facial roundness (moon face): Characteristic rounding of the face.
- Skin changes: Including thinning, bruising, and purple stretch marks (striae).
- Muscle weakness: Particularly in the legs.
- High blood pressure (hypertension).
Conn’s syndrome (excess aldosterone)
- High blood pressure: Resistant to typical treatments.
- Low potassium levels (hypokalemia): This leads to muscle cramps, weakness, and fatigue.
- Increased thirst and urination.
Virilization (excess androgens)
- Hirsutism: Increased body and facial hair.
- Deepening of voice.
- Acne and oily skin.
- Irregular menstrual periods.
Feminization (excess estrogens in men)
- Gynecomastia: Development of breast tissue.
- Loss of libido.
- Infertility.
Causes of oncocytic adrenal cortical carcinoma
The exact cause of oncocytic adrenocortical carcinoma is not well understood. However, several factors and genetic conditions have been associated with an increased risk of developing this type of cancer.
Genetic factors
- Familial cancer syndromes: Certain inherited syndromes can predispose individuals to adrenocortical carcinoma, including:
- Li-Fraumeni syndrome: Caused by mutations in the TP53 gene.
- Beckwith-Wiedemann Syndrome: A growth disorder that can increase the risk of various cancers.
- Multiple Endocrine Neoplasia Type 1 (MEN1): A genetic condition associated with tumours in endocrine glands.
Environmental and lifestyle factors
While not definitively linked, some environmental and lifestyle factors may play a role in the development of adrenocortical carcinoma:
- Exposure to carcinogens: Such as certain chemicals and radiation.
- Hormonal imbalance: Long-term use of certain medications that affect hormone levels.
Mitotic grade in oncocytic adrenocortical carcinoma
The mitotic grade refers to the number of cells undergoing mitosis (cell division) in a given amount of tissue. In oncocytic adrenocortical carcinoma, the mitotic grade measures how many cells are actively dividing in the tumour. This grade provides insight into the tumour’s aggressiveness and potential for growth.
How do pathologists measure the mitotic grade?
Pathologists measure the mitotic grade by examining tumour tissue under a microscope and counting the number of cells undergoing mitosis in a specific tissue area, usually in 10 mm2 or 50 high-powered fields (HPFs). The result is often reported as the number of mitoses per 10 mm2. For example, a report might state, “8 mitoses per 10 mm2.”
The mitotic grade is divided into two categories:
- Low mitotic grade: ≤ 20 mitotic figures per 10 mm2. These tumours are less likely to metastasize to other parts of the body.
- High mitotic grade: > 20 mitotic figures per 10 mm2. These tumours are more likely to metastasize to other parts of the body.
Why is the mitotic grade important?
The mitotic grade is important for oncocytic adrenocortical carcinoma because it helps determine the tumour’s behaviour and potential for metastatic disease. A higher mitotic grade indicates more active cell division, suggesting a more aggressive tumour that may grow and spread more rapidly. Conversely, a lower mitotic grade indicates slower growth and less aggressive tumours.
Helsinki score
The Helsinki score is a specific grading system used to evaluate the metastatic potential of adrenal cortical tumours, including oncocytic adrenocortical carcinoma. It is a composite score considering three microscopic features of the tumour: mitotic figures, necrosis, and the Ki-67 (MIB) labelling index.
The Helsinki score is calculated as follows:
- Mitotic count > 5 per 10 mm2 = 3 points.
- Necrosis = 5 points
- Ki-67 (MIB) labelling index = Numeric value of the Ki-67 index from the highest area (for example, a labelling index of 31% equals 31 points).
The score is then divided into three groups:
- Benign (noncancerous): Score 0 to 8.5.
- Malignant (cancerous): Score > 8.5.
- Malignant with poor prognosis (high risk of metastatic disease): Score > 17.
Why is the Helsinki score important?
The Helsinki score is important for several reasons:
- Diagnostic accuracy: It helps pathologists distinguish between benign and malignant adrenal cortical tumours more accurately.
- Prognosis: Provides valuable prognostic information, indicating the potential behaviour of the tumour.
- Treatment planning: Guides clinicians in deciding the appropriate course of treatment based on the tumour’s malignancy risk.
- Standardization: A standardized method for assessing adrenal cortical tumours facilitates consistent diagnoses and comparisons across different medical centres.
Tumour capsule invasion
Tumour capsule invasion occurs when cancer cells penetrate the capsule surrounding the adrenal gland. The capsule is a fibrous tissue layer that encases the adrenal gland, providing a physical barrier between the gland and surrounding tissues.
Why is tumour capsule invasion important?
- Indicator of aggressiveness: Tumour capsule invasion is a sign of aggressive tumour behaviour, indicating that the cancer cells can breach natural barriers.
- Prognosis: Capsule invasion is associated with a higher risk of recurrence and metastasis, suggesting the tumour is more likely to spread beyond the adrenal gland.
- Staging and treatment: Capsule invasion influences the staging of the tumour, which in turn affects treatment decisions. Tumours with capsule invasion may require more extensive surgical removal and closer postoperative monitoring.
Invasion of peri-adrenal adipose tissue
Peri-adrenal adipose tissue invasion occurs when cancer cells spread into the fatty tissue surrounding the adrenal gland. The peri-adrenal adipose tissue is a cushion for the adrenal gland, and its invasion by tumour cells indicates a more advanced stage of disease.
Why is peri-adrenal adipose tissue invasion important?
- Marker of advanced disease: Invasion into the peri-adrenal adipose tissue signifies a more advanced and aggressive tumour. It indicates that the cancer is not confined to the adrenal gland and has begun to spread to nearby structures.
- Impact on prognosis: Peri-adrenal adipose tissue invasion is associated with a worse prognosis. It increases the likelihood of metastasis and recurrence.
- Surgical considerations: Tumours with peri-adrenal adipose tissue invasion often require more extensive surgical resection to ensure complete removal of cancerous tissue.
- Follow-up and monitoring: Patients with this type of invasion need careful postoperative monitoring for signs of recurrence and distant metastasis.
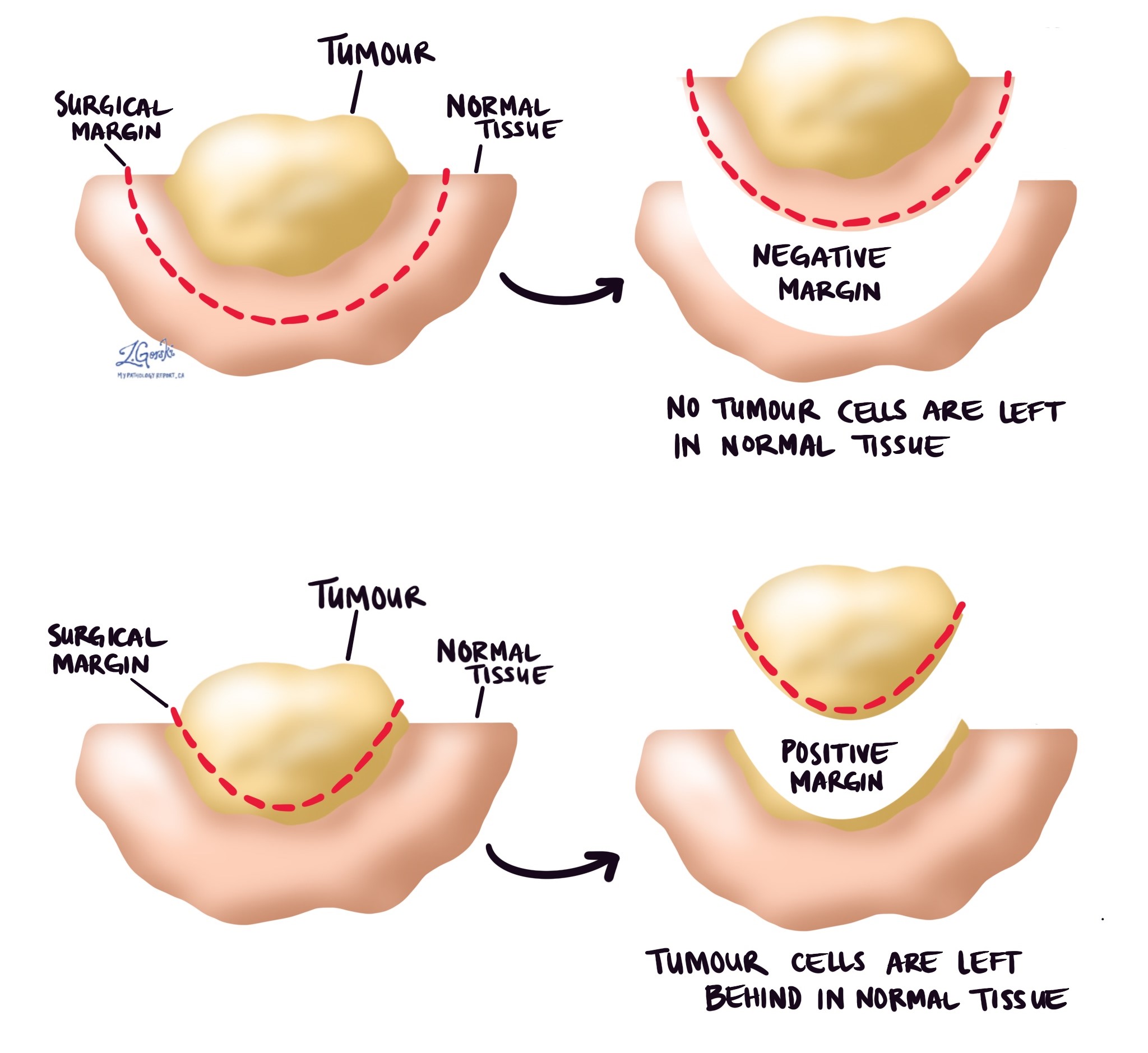
Margins
In pathology, a margin refers to the border of healthy tissue surrounding a tumour removed during surgery. When a tumour is excised, the goal is to remove the tumour itself and some surrounding tissue to ensure that no cancer cells are left behind. A pathologist then examines the tissue removed to determine if the margins are free of cancer cells.
Types of margins:
- Negative (clear) margin: No cancer cells are found at the edge of the removed tissue, indicating that the tumour has been entirely excised.
- Positive margin: Cancer cells are present at the edge of the removed tissue, suggesting that some of the tumour may still be left in the body.
- Close margin: Cancer cells are near the edge but not at the edge itself, which may indicate a higher risk of residual disease.
Why are margins important?
- Complete tumour removal: Ensuring that the margins are free of cancer cells is critical to achieving complete tumour removal. This is particularly important for oncocytic adrenal cortical carcinoma due to its aggressive nature and potential for recurrence.
- Prognosis: Clear margins are associated with a better prognosis and a lower risk of recurrence. Conversely, positive margins are associated with a higher likelihood of the cancer returning.
- Guiding further treatment: If positive or close margins are detected, additional treatments such as re-excision, radiation therapy, or systemic therapies may be necessary to address any remaining cancer cells.
- Reducing recurrence risk: Clear margins reduce the risk of local recurrence, which is crucial for improving long-term survival outcomes.