by Jason Wasserman MD PhD FRCPC and Trevor Flood MD
October 11, 2022
What is Xp11 translocation renal cell carcinoma?
Xp11 translocation renal cell carcinoma (RCC) is a type of kidney cancer. It is called ‘Xp11 translocation’ because the tumour cells contain a genetic alteration involving the TFE3 gene which is located in the p11 region of chromosome X. Another name for this type of cancer is TFE3-rearranged renal cell carcinoma.
What causes Xp11 translocation renal cell carcinoma?
This type of cancer is associated with an alteration in the TFE3 gene. This alteration results in the activation of other genes that lead to cell growth and cell division. What causes the initial change in the TFE3 gene is still not known.
What are the symptoms of Xp11 translocation renal cell carcinoma?
Patients with small tumours may not notice any symptoms. As the tumour grows, symptoms may include pain in the back or side of the body and bloody urine.
How is the diagnosis of Xp11 translocation renal cell carcinoma made?
For most patients, the diagnosis of Xp11 translocation RCC is made after the entire tumour is surgically removed and the tissue is sent to a pathologist for examination under a microscope. After examining the tissue, your pathologist will perform additional tests to confirm the presence of an alteration involving the TFE3 gene. Currently used tests to identify this alteration include immunohistochemistry, fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS).
Immunohistochemistry
Immunohistochemistry is a test that allows pathologists to see chemicals such as proteins inside cells. When immunohistochemistry is performed, the tumour cells in Xp11 translocation RCC will show strong expression of the TFE3 protein in a part of the cell called the nucleus. Pathologists describe this pattern as ‘nuclear reactivity’ or ‘nuclear expression’.
Fluorescence in situ hybridization
Xp11 translocation RCC results from a genetic change called a fusion between the TFE3 gene and a partner gene. For Xp11 translocation RCC, the possible partner genes include ASPSCR1 (ASPL), PRCC, NONO (P54NRB), SFPQ (PSF), RBM10, MED15, CLTC, DVL2, PARP14, KAT6A, NEAT1, MATR3, FUBP1, and EWSR1. Fluorescence in situ hybridization (FISH) is a molecular test that allows pathologists to see genetic changes such as fusions. Identifying a fusion involving the TFE3 gene confirms the diagnosis of TFE3-rearranged RCC.
What is the WHO/ISUP grade and why is it important?
Pathologists divide Xp11 translocation RCC into four grades using a system developed by the World Health Organization (WHO) and the International Society for Urological Pathology (ISUP). Prior to 2016, these tumour types were graded using Fuhrman nuclear grading system. The WHO/ISUP grading system and the Fuhrman nuclear grading system are similar and both employ a scoring system from 1 to 4. The WHO/ISUP grade is based on microscopic features of the tumour cells, in particular, the size and shape of the tumour cell nuclei and the presence of nucleoli.
The WHO/ISUP grade is important because it is can predict the future behaviour of the tumour. In general, high-grade tumours (WHO/ISUP grades 3 and 4) are associated with a worse prognosis than low-grade tumours (WHO/ISUP grades 1 and 2) and are more likely to spread to other parts of the body.
The WHO/ISUP grading system:
- Grade 1 – The tumour cell nuclei are small and round. The nucleoli are difficult to see even when the cells are examined with a high-magnification lens.
- Grade 2 – The tumour cell nuclei are slightly larger and irregularly shaped. Nucleoli are easier to see but only after the cells are examined with a high-magnification lens.
- Grade 3 – The tumour cell nuclei are obviously irregular and enlarged. The nucleoli are very easy to see even when the cells are examined with a low-magnification lens.
- Grade 4 – The tumour cell nuclei are bizarre, extremely irregular and often multilobed. Tumours with sarcomatoid and rhabdoid cells are included in this category (see sections below for more details).
What are sarcomatoid cells and why are they important?
Sarcomatoid cells are tumour cells that have changed both their shape and their behaviour. Sarcomatoid tumour cells can be found in almost all types of renal cell carcinoma, including Xp11 translocation RCC. Instead of being round in shape, the sarcomatoid cells are now long and thin. Pathologists describe cells with this shape as spindle cells. Tumours with sarcomatoid cells are considered high grade (see WHO/ISUP grade above) and they are associated with a worse prognosis.
What are rhabdoid cells and why are they important?
Rhabdoid cells are tumour cells that have changed to look more like muscle cells. Rhabdoid tumour cells can be found in almost all types of renal cell carcinoma, including Xp11 translocation RCC. Tumours with rhabdoid cells are considered high grade (see WHO/ISUP grade above) and they are associated with a worse prognosis.
What if more than one tumour is found in the kidney?
Sometimes, more than one tumour is found in the same kidney. When only one tumour is found, pathologists call this unifocal. When more than one tumour is found, pathologists call this multifocal. When multiple tumours are found, they are usually of the same type. For example, they are all Xp11 translocation RCCs. However, different types of tumours can also be found in the same kidney. In that case, your report will list and describe each type of tumour found.
What does tumour necrosis mean?
Necrosis is a form of cell death and it commonly occurs in cancerous tumours. Your pathologist will closely examine the tumour for evidence of necrosis. The presence of necrosis is important because it is associated with a worse prognosis.
What does tumour extension mean and why is it important?
The normal kidney sits near the back of the body and is surrounded by fat. The adrenal gland sits directly above the kidney and the bladder is attached to the kidney by a long thin tube called the ureter which connects to the kidney in a region called the ‘renal sinus’. Xp11 translocation RCC starts inside the kidney but as it grows, it can extend into any of these structures and organs. The growth of the tumour into surrounding organs is called tumour extension.
Your pathologist will carefully examine the specimen for any evidence of tumour extension and all structures or organs involved will be listed in your report. Tumour extension into any of these structures or organs is important because it is associated with a worse prognosis and it is also used to determine the pathologic stage (see Pathologic stage below).
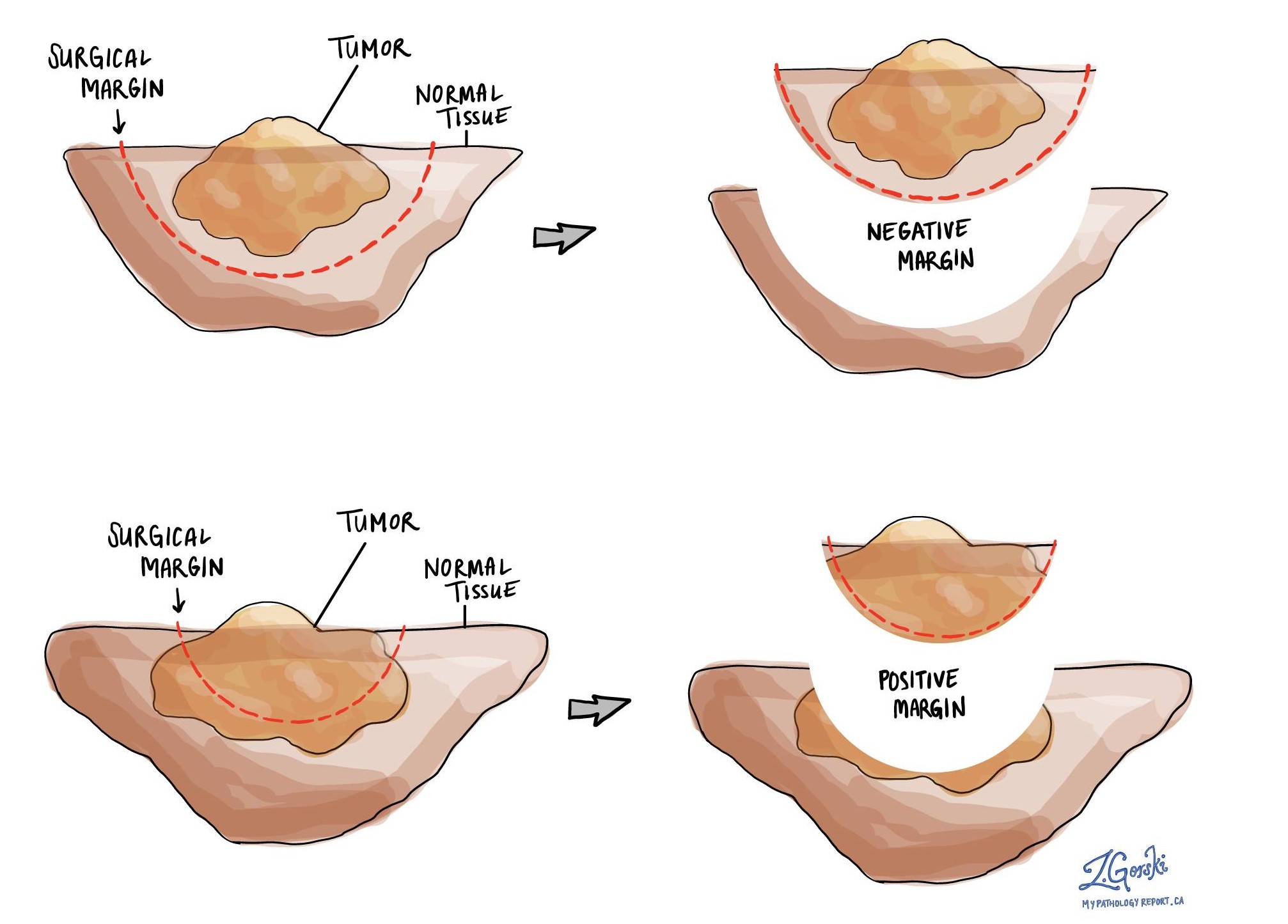
What is a margin?
In pathology, a margin is the edge of a tissue that is cut when removing a tumour from the body. The margins described in a pathology report are very important because they tell you if the entire tumour was removed or if some of the tumour was left behind. The margin status will determine what (if any) additional treatment you may require.
Most pathology reports only describe margins after a surgical procedure called an excision or resection has been performed for the purpose of removing the entire tumour. For this reason, margins are not usually described after a procedure called a biopsy is performed for the purpose of removing only part of the tumour.
If only part of the kidney was removed (a procedure known as a ‘partial nephrectomy’), the margins will include the fat surrounding that portion of the kidney and the area where the kidney was divided. If the entire kidney was removed (a procedure known as a ‘total’ or ‘radical nephrectomy’) the margins will include the fat surrounding the kidney, the ureter (the tube that connects the kidney to the bladder), and some large blood vessels (usually arteries and veins). Some larger specimens may include additional margins.
Pathologists carefully examine the margins to look for tumour cells at the cut edge of the tissue. If tumour cells are seen at the cut edge of the tissue, the margin will be described as positive. If no tumour cells are seen at the cut edge of the tissue, a margin will be described as negative. Even if all of the margins are negative, some pathology reports will also provide a measurement of the closest tumour cells to the cut edge of the tissue.
A positive (or very close) margin is important because it means that tumour cells may have been left behind in your body when the tumour was surgically removed. For this reason, patients who have a positive margin may be offered another surgery to remove the rest of the tumour or radiation therapy to the area of the body with the positive margin. The decision to offer additional treatment and the type of treatment options offered will depend on a variety of factors including the type of tumour removed and the area of the body involved. For example, additional treatment may not be necessary for a benign (non-cancerous) type of tumour but may be strongly advised for a malignant (cancerous) type of tumour.
A negative margin means no cancer cells were seen at the cut edge of the tissue. In contrast, a positive margin means that cancer cells are seen at the cut edge of the tissue. Your pathologist will report any positive margins and the location of that margin. A positive margin is associated with an increased risk of the tumour coming back in the same area of the body.
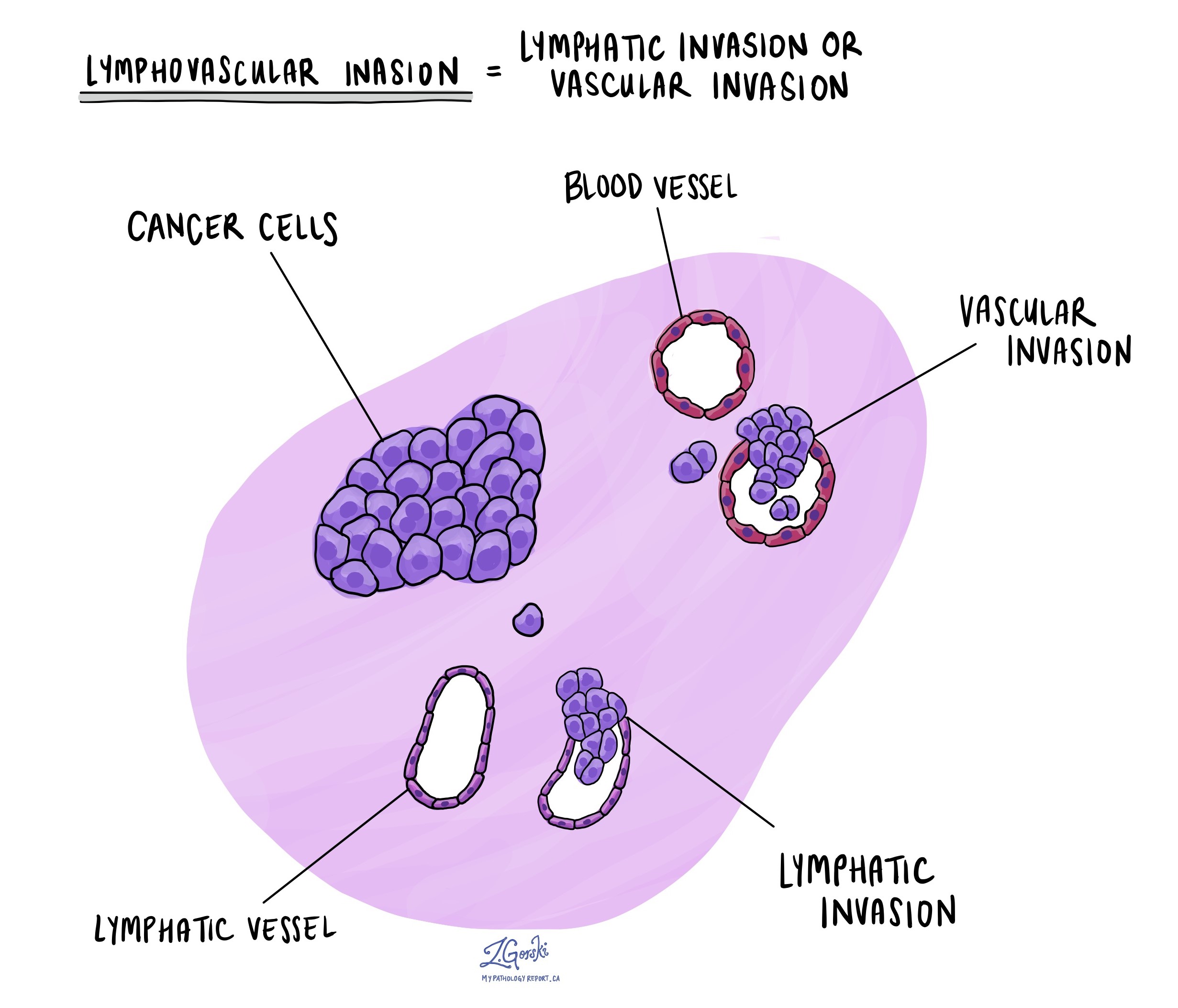
What does lymphovascular invasion mean and why is it important?
Lymphovascular invasion means that cancer cells were seen inside a blood vessel or lymphatic vessel. Blood vessels are long thin tubes that carry blood around the body. Lymphatic vessels are similar to small blood vessels except that they carry a fluid called lymph instead of blood. The lymphatic vessels connect with small immune organs called lymph nodes that are found throughout the body. Lymphovascular invasion is important because cancer cells can use blood vessels or lymphatic vessels to spread to other parts of the body such as lymph nodes or the lungs.
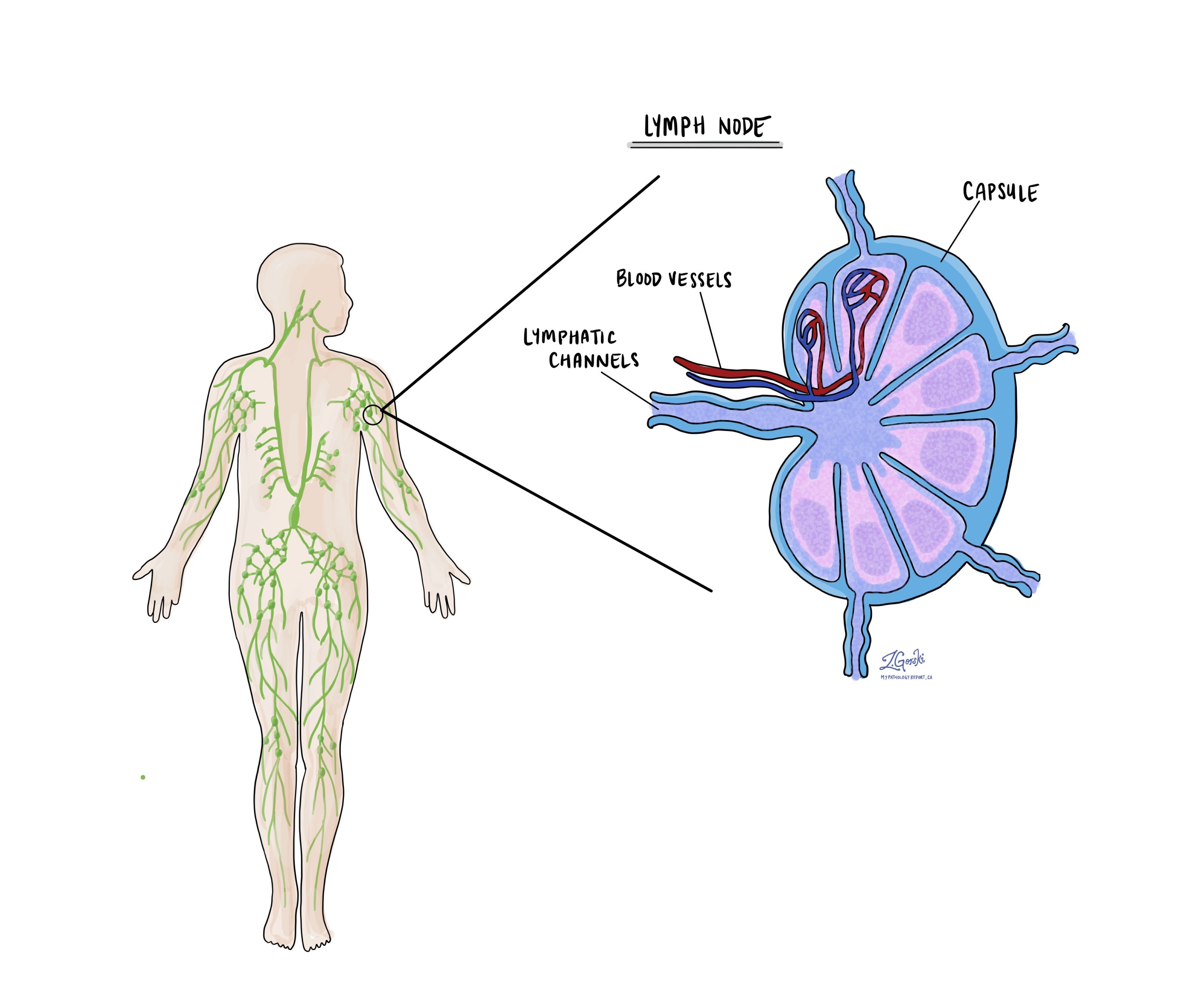
Were lymph nodes examined and did any contain cancer cells?
Lymph nodes are small immune organs found throughout the body. Cancer cells can spread from a tumour to lymph nodes through small vessels called lymphatics. For this reason, lymph nodes are commonly removed and examined under a microscope to look for cancer cells. The movement of cancer cells from the tumour to another part of the body such as a lymph node is called metastasis.
Cancer cells typically spread first to lymph nodes close to the tumour although lymph nodes far away from the tumour can also be involved. For this reason, the first lymph nodes removed are usually close to the tumour. Lymph nodes further away from the tumour are only typically removed if they are enlarged and there is a high clinical suspicion that there may be cancer cells in the lymph node.
If any lymph nodes were removed from your body, they will be examined under the microscope by a pathologist and the results of this examination will be described in your report. Most reports will include the total number of lymph nodes examined, where in the body the lymph nodes were found, and the number (if any) that contain cancer cells. If cancer cells were seen in a lymph node, the size of the largest group of cancer cells (often described as “focus” or “deposit”) will also be included.
The examination of lymph nodes is important for two reasons. First, this information is used to determine the pathologic nodal stage (pN). Second, finding cancer cells in a lymph node increases the risk that cancer cells will be found in other parts of the body in the future. As a result, your doctor will use this information when deciding if additional treatment such as chemotherapy, radiation therapy, or immunotherapy is required.
What does it mean if a lymph node is described as positive?
Pathologists often use the term “positive” to describe a lymph node that contains cancer cells. For example, a lymph node that contains cancer cells may be called “positive for malignancy” or “positive for metastatic carcinoma”.
What does it mean if a lymph node is described as negative?
Pathologists often use the term “negative” to describe a lymph node that does not contain any cancer cells. For example, a lymph node that does not contain cancer cells may be called “negative for malignancy” or “negative for metastatic carcinoma”.
What does extranodal extension mean?
All lymph nodes are surrounded by a thin layer of tissue called a capsule. Extranodal extension means that cancer cells within the lymph node have broken through the capsule and have spread into the tissue outside of the lymph node. Extranodal extension is important because it increases the risk that the tumour will regrow in the same location after surgery. For some types of cancer, extranodal extension is also a reason to consider additional treatment such as chemotherapy or radiation therapy.

What is the pathologic stage (pTNM) for Xp11 translocation renal cell carcinoma?
The pathologic stage for Xp11 translocation RCC is based on the TNM staging system, an internationally recognized system originally created by the American Joint Committee on Cancer. This system uses information about the primary tumour (T), lymph nodes (N), and distant metastatic disease (M) to determine the complete pathologic stage (pTNM). Your pathologist will examine the tissue submitted and give each part a number. In general, a higher number means more advanced disease and a worse prognosis.
Tumour stage (pT) for Xp11 translocation renal cell carcinoma
Xp11 translocation RCC is given a tumour stage between 1 and 4 based on the size of the tumour and the growth of the tumour into organs attached to the kidney.
- T1 – The tumour is less than or equal to 7 centimetres and is still entirely within the kidney.
- T2 – The tumour is greater than 7 centimetres but is still entirely within the kidney.
- T3 – The tumour is grown into the fat around the kidney or into a large vein attached to the kidney.
- T4 – The tumour has grown well outside the kidney and through a barrier known as ‘Gerota’s fascia’ OR into the adrenal gland above the kidney.
Nodal stage (pN) for Xp11 translocation renal cell carcinoma
Xp11 translocation RCC is given a nodal stage of 0 or 1 based on the presence of tumour cells in a lymph node. If no lymph nodes are involved the nodal stage is N0. If any tumour cells are seen in a lymph node the nodal stage is N1. If no lymph nodes are submitted for pathological examination, the nodal stage cannot be determined and the nodal stage is listed as NX.
Metastatic stage (pM) for Xp11 translocation renal cell carcinoma
Xp11 translocation RCC is given a metastatic stage of 0 or 1 based on the presence of tumour cells at a distant site in the body (for example the lungs). The metastatic stage can only be determined if tissue from a distant site is submitted for pathological examination. Because this tissue is rarely present, the metastatic stage cannot be determined and is listed as MX.
Pathologic findings in the non-neoplastic kidney
The non-neoplastic kidney is the tissue outside of the tumour. Your pathologist will carefully examine the non-neoplastic tissue for evidence of other diseases that can commonly affect the kidney such as arterionephrosclerosis (high blood pressure) and diabetic nephropathy (diabetes).



