by Jason Wasserman MD PhD FRCPC
July 20, 2025
Mucinous adenocarcinoma is a type of colon cancer. It is called “mucinous” because at least 50% of the tumour is made up of extracellular mucin. Mucin is a gel-like substance that is normally produced by the cells that line the inside of the colon. When produced in large amounts by cancer cells, mucin can form large pools that separate and surround the tumour cells.
Mucinous adenocarcinoma accounts for about 10 to 20% of all colon cancers in adults. Compared to other types of colon cancer, mucinous adenocarcinoma is more likely to be associated with genetic changes in the mismatch repair system, which is responsible for fixing errors in DNA. These changes can increase the risk of developing cancer.
What causes mucinous adenocarcinoma?
The exact cause of mucinous adenocarcinoma is not known. However, like most colon cancers, this type of tumour develops when cells in the lining of the colon begin to grow abnormally. This abnormal growth is usually the result of changes in genes that control cell division and repair. In some people, these changes may be inherited, such as in Lynch syndrome, which affects the mismatch repair system. In other cases, they are acquired over time and may be influenced by lifestyle factors, inflammation, or environmental exposures.
What are the symptoms of mucinous adenocarcinoma?
Many people with mucinous adenocarcinoma do not experience symptoms until the tumour has grown large or spread to other parts of the body. When symptoms do occur, they may include:
-
Changes in bowel habits such as diarrhea, constipation, or narrowing of the stool.
-
Blood in the stool or rectal bleeding.
-
Abdominal pain or cramping.
-
Unexplained weight loss.
-
Fatigue or weakness.
These symptoms can also be caused by other conditions, so further testing is usually needed to make a diagnosis.
How is this diagnosis made?
The diagnosis of mucinous adenocarcinoma is usually made after a small tissue sample is removed from the colon or rectum during a colonoscopy. The procedure used to collect the sample may be called a biopsy or polypectomy. The tissue is then examined under a microscope by a pathologist.
Once the entire tumour is removed, your pathologist will look for additional features such as tumour grade, invasion into surrounding tissues, lymphovascular and perineural invasion, tumour budding, cancer cells in lymph nodes, and tumour deposits. Tests to assess for mismatch repair deficiency may also be performed.
What does mucinous adenocarcinoma look like under the microscope?
Under the microscope, mucinous adenocarcinoma is made up of groups of tumour cells floating in large areas of extracellular mucin. The tumour cells may form round structures called glands, or they may appear as nests or sheets. Some tumour cells may contain mucin inside the cell, pushing the nucleus to the side. These cells are called signet ring cells, and they are commonly seen in mucinous adenocarcinoma.
How do pathologists grade mucinous adenocarcinoma?
Tumour grade describes how closely the tumour cells resemble normal cells. In mucinous adenocarcinoma, grade is based on how much of the tumour forms gland-like structures:
-
Grade 1 (well differentiated): more than 95% of the tumour forms glands.
-
Grade 2 (moderately differentiated): 50–95% of the tumour forms glands.
-
Grade 3 (poorly differentiated): less than 50% of the tumour forms glands.
-
Grade 4 (undifferentiated): no gland formation.
Higher-grade tumours behave more aggressively and are more likely to spread.
Level of invasion
Invasion refers to how deeply the tumour has grown into the wall of the colon. A mucinous adenocarcinoma starts from gland-forming cells located in a thin layer of tissue on the inner surface of the colon called the mucosa. As the tumour grows, it can spread beyond the mucosa into deeper layers of tissue.
Beneath the mucosa are several important layers of the colon wall:
-
Submucosa – a supportive layer made of connective tissue located just beneath the mucosa.
-
Muscularis propria – a thick layer of muscle that helps move waste through the colon.
-
Subserosal tissue – a layer of fat beneath the muscle.
-
Serosa – the outermost layer that covers the outside surface of the colon.
As a mucinous adenocarcinoma grows, it can invade into these deeper layers. In more advanced cases, the tumour may grow all the way through the wall of the colon and spread directly into nearby organs or tissues.
The level of invasion refers to the deepest layer that the tumour has reached. This can only be determined after the tumour is examined under the microscope by a pathologist. The level of invasion is very important because tumours that grow deeper into the colon wall are more likely to spread to lymph nodes or other parts of the body. The level of invasion is also used to determine the pathologic tumour stage (pT), which helps doctors plan further treatment and estimate the risk of the cancer coming back.
Tumour budding
Tumour budding refers to small clusters or single cancer cells seen at the edge of the tumour. The number of buds seen under the microscope is used to assign a score: low, intermediate, or high. A high tumour budding score is associated with a greater risk of the cancer spreading.
Lymphatic invasion
Lymphatic invasion means that cancer cells have entered small vessels called lymphatic channels, which are part of the body’s immune and drainage system. These channels are lined by thin endothelial cells and usually do not contain red blood cells. When tumour cells are found inside them, it suggests a higher chance that the cancer may spread to nearby lymph nodes. Sometimes, additional special stains (immunohistochemistry) are used by pathologists to identify cancer cells in these tiny vessels.
Vascular invasion
Vascular invasion means that cancer cells have entered blood vessels. This can happen inside the wall of the colon (intramural vascular invasion) or outside the wall in surrounding tissues (extramural vascular invasion). Both types are associated with a higher risk that the cancer will spread to other parts of the body, such as the lungs or liver, but extramural vascular invasion is considered particularly important.
In some cases, pathologists may use special stains to highlight the blood vessels and confirm the presence of tumour cells inside them. Recognizing this type of invasion helps guide treatment decisions and provides important information about prognosis.
Perineural invasion
Perineural invasion means that cancer cells are growing along or around a nerve. This is most often seen in advanced tumours and is associated with a higher risk of the cancer spreading or coming back after treatment. Under the microscope, pathologists look for tumour cells that wrap around at least one-third of the outside surface of a nerve. Perineural invasion can occur in any part of the colon and is more common when other high-risk features, such as vascular invasion or lymphatic invasion, are also present.
Immune response
The body’s immune system often tries to fight back against cancer by sending immune cells like lymphocytes to the area around the tumour. When a strong immune response is seen under the microscope, it may be a sign that the body is trying to contain or slow the growth of the cancer. A particular type of immune response called a Crohn-like reaction involves groups of immune cells clustered near the tumour and is generally associated with a better outcome.
In some cases, pathologists may use immunohistochemistry to assess specific types of immune cells in the tissue. While not always reported in standard pathology reports, immune response is increasingly being recognized as an important part of cancer biology.
Margins
Margins refer to the edges of tissue removed during surgery. In colon cancer surgery, pathologists examine different types of margins:
- Proximal and distal margins: These are the ends of the colon segment removed during surgery.
- Circumferential resection margin (CRM): The outermost edge of the resected tissue, which is particularly important in rectal cancer.
- Mesocolic margin: This margin is important for tumours in the cecum. A margin is considered positive if cancer cells are found at the edge, meaning some cancer may have been left behind. A negative margin means no cancer was found at the edge, indicating the tumour was removed entirely.
Treatment effect
The tumour may shrink or disappear completely if a patient receives treatment before surgery, such as chemotherapy or radiation therapy. Pathologists assess the tumour to determine how much remains after treatment.
The response is classified as:
- No viable cancer cells (complete response, score 0).
- Single cells or rare small groups of cancer cells (near complete response, score 1).
- Residual cancer with evidence of tumour regression, but more than single cells or rare small groups (partial response, score 2).
- Extensive residual cancer with no evident tumour regression (poor or no response, score 3).
Tumour deposits
Tumour deposits are small groups of cancer cells found in the fat surrounding the colon or rectum. They are located in the lymphatic drainage area of the primary tumour but do not contain identifiable lymph node tissue or blood vessels. If a tumour focus is found in a vessel, it is classified as vascular invasion rather than a tumour deposit. Similarly, if a tumour focus is found near a nerve, it is classified as perineural invasion.
Tumour deposits are important because their presence increases the risk of cancer spreading. If tumour deposits are present but no lymph nodes contain cancer, the cancer is classified as N1c, regardless of the tumour (T) stage. If positive lymph nodes are also found, the cancer is classified based on the number of involved lymph nodes (N1a or N1b), but the presence and number of tumour deposits are still noted in the pathology report. Tumour deposits are considered an adverse prognostic factor, and their presence may influence treatment decisions, such as the need for chemotherapy.
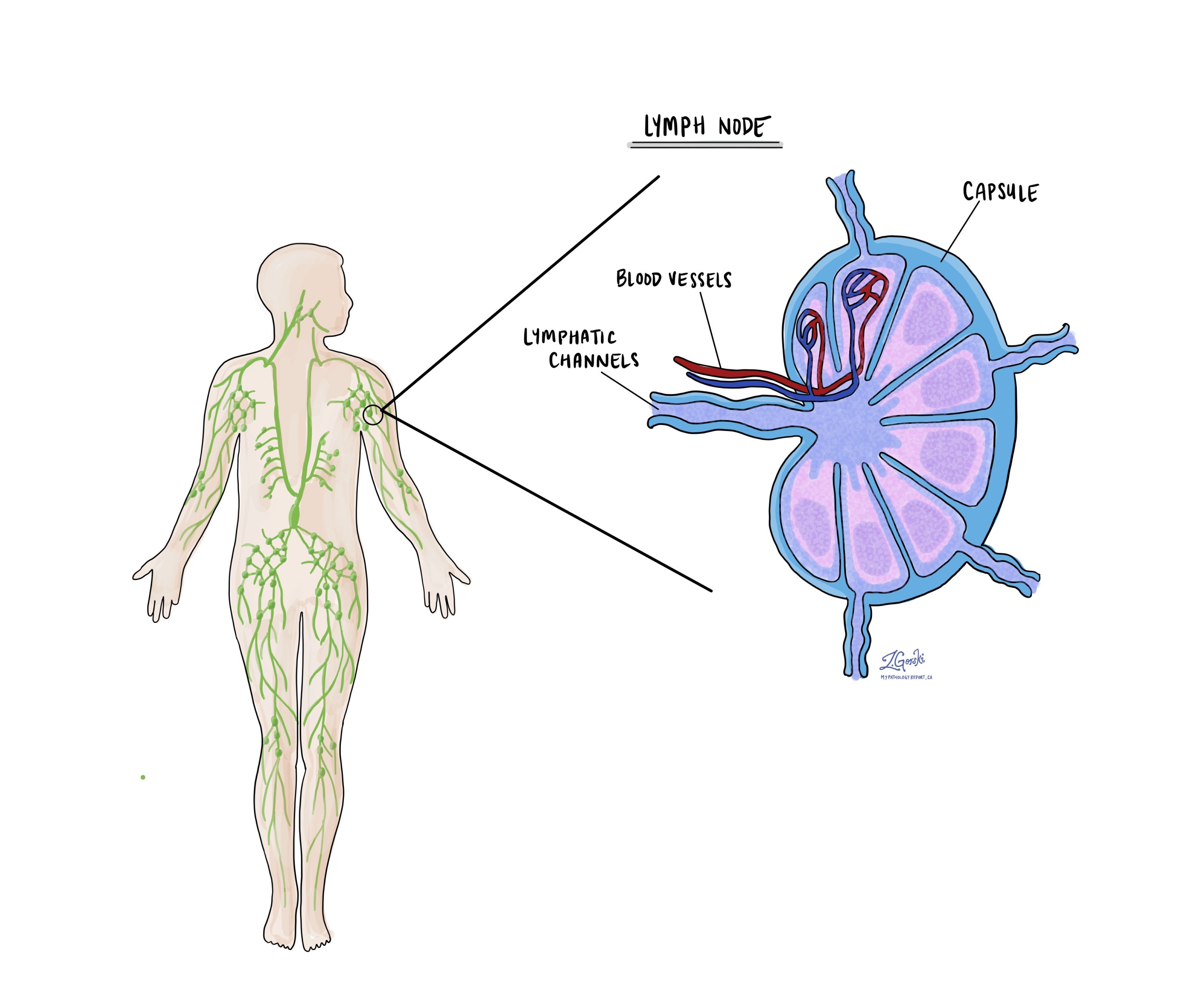
Lymph nodes
Lymph nodes are small immune organs found throughout the body. Cancer cells can spread from a tumour to these nodes via lymphatic vessels, prompting doctors to remove and examine them for cancer. This process is known as metastasis. Typically, cancer cells first migrate to lymph nodes nearest to the tumour, but more distant nodes can also be affected. Surgeons often remove the closest lymph nodes first and may take additional ones if they appear enlarged and potentially cancerous.
Pathologists examine the removed lymph nodes and report results as “positive” if cancer cells are found and “negative” if not. If cancer is detected, the report may indicate the size of the largest cluster, referred to as a “focus” or “deposit.” Extranodal extension refers to tumour cells breaking through the lymph node’s outer capsule into nearby tissue.
Examining lymph nodes is important for determining the pathologic nodal stage (pN) and gauging the risk of cancer spreading to other body parts. This information helps doctors determine whether further treatments, such as chemotherapy, radiation, or immunotherapy, are necessary.
Molecular markers
Molecular markers are changes in specific genes or proteins found in cancer cells that can provide important information about how a tumour behaves and how it may respond to certain treatments. These changes are identified through specialized laboratory tests performed on the tumour tissue. The results of these tests can help guide treatment decisions, especially in cases where the disease is advanced or has spread to other parts of the body.
Mismatch repair protein testing
Mismatch repair (MMR) proteins are part of the cell’s natural system for fixing DNA damage. These proteins—MLH1, PMS2, MSH2, and MSH6—work in pairs to repair small errors that occur when cells divide. If one of these proteins is missing, the repair system does not work properly, and this can lead to cancer.
Pathologists use a test called immunohistochemistry to check if these proteins are present in the tumour. If one or more proteins are missing, this is called mismatch repair deficiency. Mismatch repair deficiency may be caused by an inherited condition such as Lynch syndrome or by a non-inherited (sporadic) change in the tumour. If the proteins are present, the result is called mismatch repair proficient, meaning the repair system appears to be working normally.
Finding mismatch repair deficiency is important because it may suggest a higher risk of other cancers in the patient or their family. It can also help doctors decide whether certain treatments, like immunotherapy, might be helpful.
MLH1 promoter hypermethylation
One common reason for mismatch repair deficiency is a change called MLH1 promoter hypermethylation. This is not a mutation in the gene itself, but a chemical change that turns off the MLH1 gene so it cannot make its protein. When MLH1 is turned off, its partner protein PMS2 is also lost, and the DNA repair system stops working.
To figure out why MLH1 is missing, doctors may test for a specific mutation in another gene called BRAF. If a BRAF mutation (specifically V600E) is found, it suggests that the tumour is sporadic, meaning it developed on its own and is not due to an inherited condition like Lynch syndrome.
In some cases, doctors may test directly for MLH1 hypermethylation. If this is found, it also supports the diagnosis of a sporadic tumour. These tests help doctors decide whether genetic testing for Lynch syndrome is needed.
KRAS and NRAS
KRAS and NRAS are genes that help control how cells grow and divide. Changes (called mutations) in these genes are common in colorectal cancer. If a tumour has a mutation in either KRAS or NRAS, it is unlikely to respond to targeted treatments such as cetuximab or panitumumab, which are drugs that block a protein called EGFR. For this reason, testing for KRAS and NRAS mutations is usually done before starting one of these therapies. The test looks at specific parts of the genes known as codons, especially codons 12, 13, 61, and 146. About half of all colorectal cancers have a mutation in either KRAS or NRAS.
BRAF
BRAF is another gene that works in the same pathway as KRAS and NRAS to help regulate cell growth. A specific mutation in BRAF, called V600E, is sometimes found in colorectal cancers, including mucinous adenocarcinomas. This mutation is associated with a more aggressive tumour and a higher chance of spreading. BRAF testing can also help determine whether the cancer might be related to Lynch syndrome. If the V600E mutation is found, Lynch syndrome is much less likely. Similar to KRAS and NRAS mutations, tumours with a BRAF mutation usually do not respond well to anti-EGFR therapy.
PIK3CA
PIK3CA is a gene that helps cells grow and survive. Mutations in PIK3CA are found in about 10 to 20 percent of colorectal cancers. These mutations may reduce how well the cancer responds to anti-EGFR therapy, especially when they occur alongside KRAS or NRAS mutations. Some studies suggest that patients with a PIK3CA mutation may benefit from taking aspirin (acetylsalicylic acid) after surgery, but this is still being researched and is not yet a standard part of treatment.
PTEN
PTEN is a gene that helps control the same growth pathway as PIK3CA. When PTEN is not working properly, tumour cells may grow more easily and may be less likely to respond to anti-EGFR treatment. Pathologists can check for PTEN loss either by testing the gene itself or by using a test called immunohistochemistry to see if the PTEN protein is missing in the tumour cells. PTEN loss is sometimes found in tumours that also have mutations in KRAS, BRAF, or PIK3CA.
EGFR
EGFR stands for epidermal growth factor receptor. It is a protein found on the surface of many cancer cells that helps them grow and divide. Drugs like cetuximab and panitumumab work by blocking EGFR to slow tumour growth. However, EGFR testing is not routinely used in colorectal cancer to decide whether these drugs will be helpful. Instead, treatment decisions are usually based on whether the tumour has mutations in KRAS or NRAS, since these mutations are more reliable indicators of how the tumour will respond to anti-EGFR therapy.
PD-L1
PD-L1 is a protein that some cancer cells use to hide from the immune system. A test for PD-L1 may be done to help identify patients who could benefit from immunotherapy. The test result is often given as a CPS score, which stands for Combined Positive Score. This score compares the number of tumour and immune cells producing PD-L1 to the total number of tumour cells. A higher CPS score means more cells are expressing PD-L1, which in some cancers may suggest a better response to immunotherapy. However, the usefulness of PD-L1 testing in colorectal cancer is still being studied and is not currently a routine part of treatment planning.
HER2
HER2, also called ERBB2, is a gene commonly tested in breast and stomach cancers, but it can also be relevant in colorectal cancer. Some colorectal cancers show HER2 overexpression or amplification, particularly those that do not have mutations in KRAS or BRAF. In certain situations, HER2-positive colorectal cancers may respond to HER2-targeted therapies. HER2 testing may be considered when other treatments are no longer effective or as part of a clinical trial.
Pathologic stage (pTNM)
Pathologic staging for mucinous adenocarcinoma of the colon describes how far the cancer has spread in your body at the time of surgery. It is based on the examination of tissue under the microscope by a pathologist after the tumor has been removed.
The system uses three key components:
-
T (Tumor): How deep the tumor has grown into the wall of the colon or surrounding areas.
-
N (Nodes): Whether the cancer has spread to nearby lymph nodes.
-
M (Metastasis): Whether the cancer has spread to distant parts of the body (this is usually assessed by imaging and not by the pathologist).
The sections below focus on the T and N stages, which are determined by the pathologist.
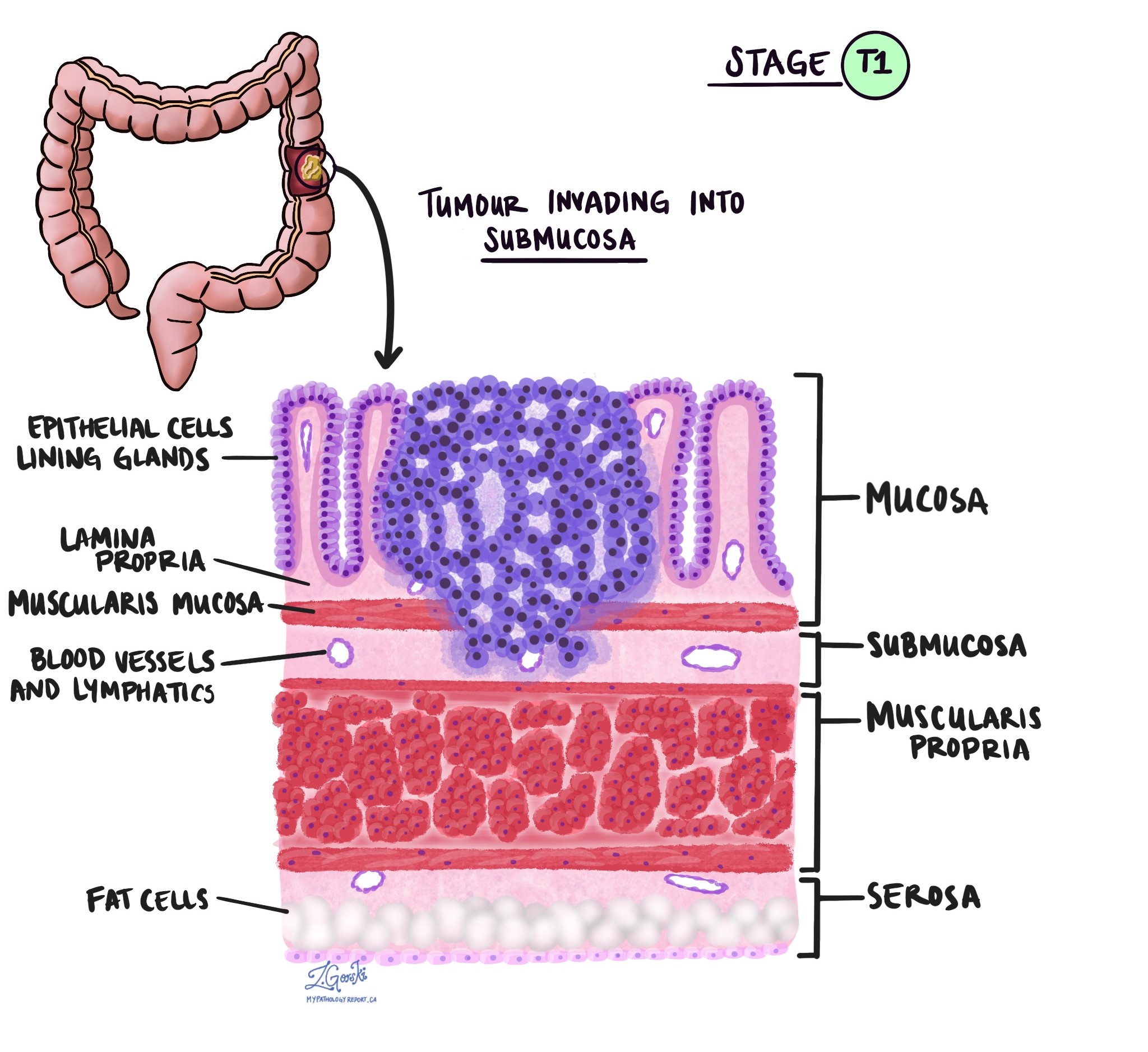
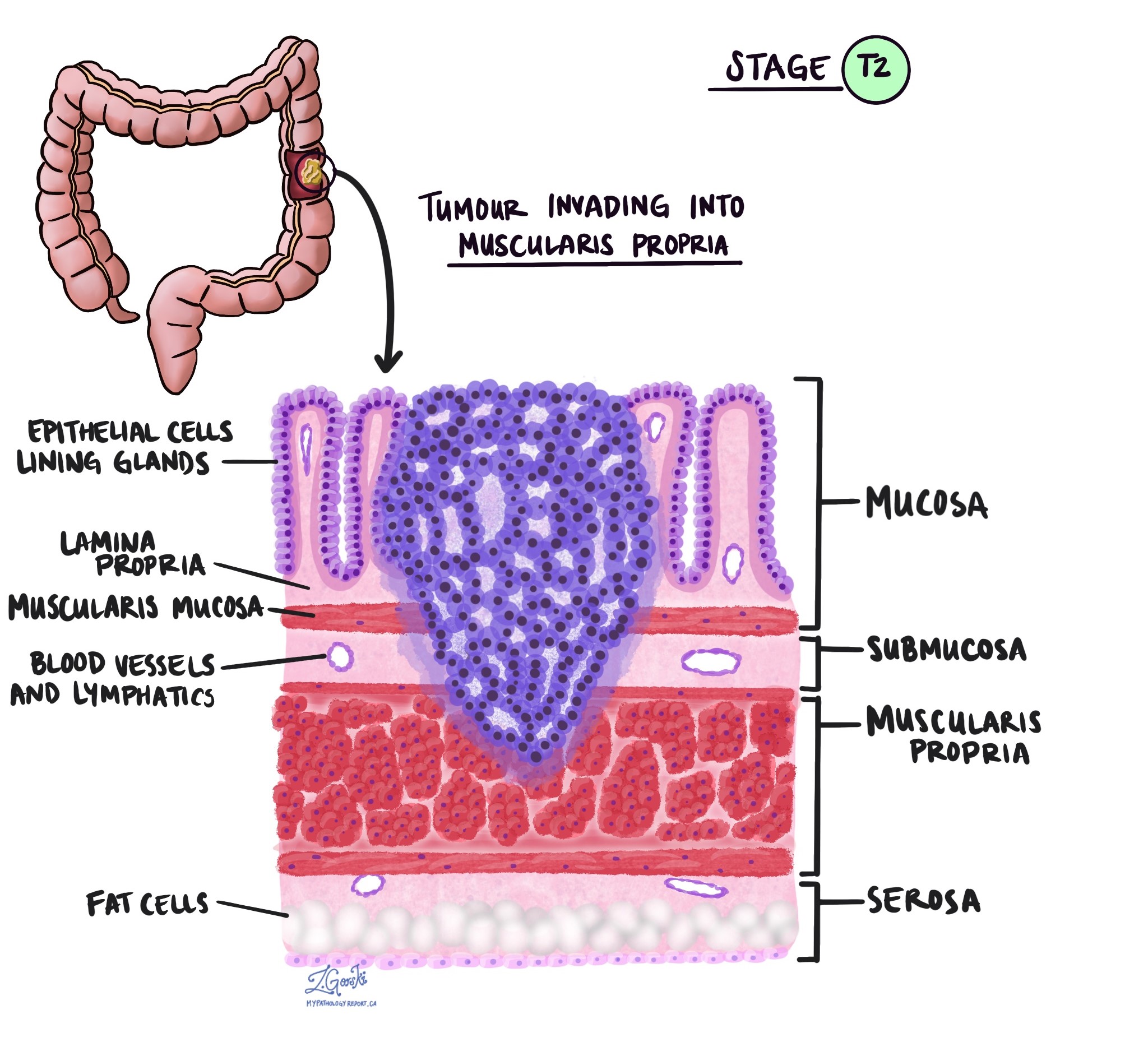
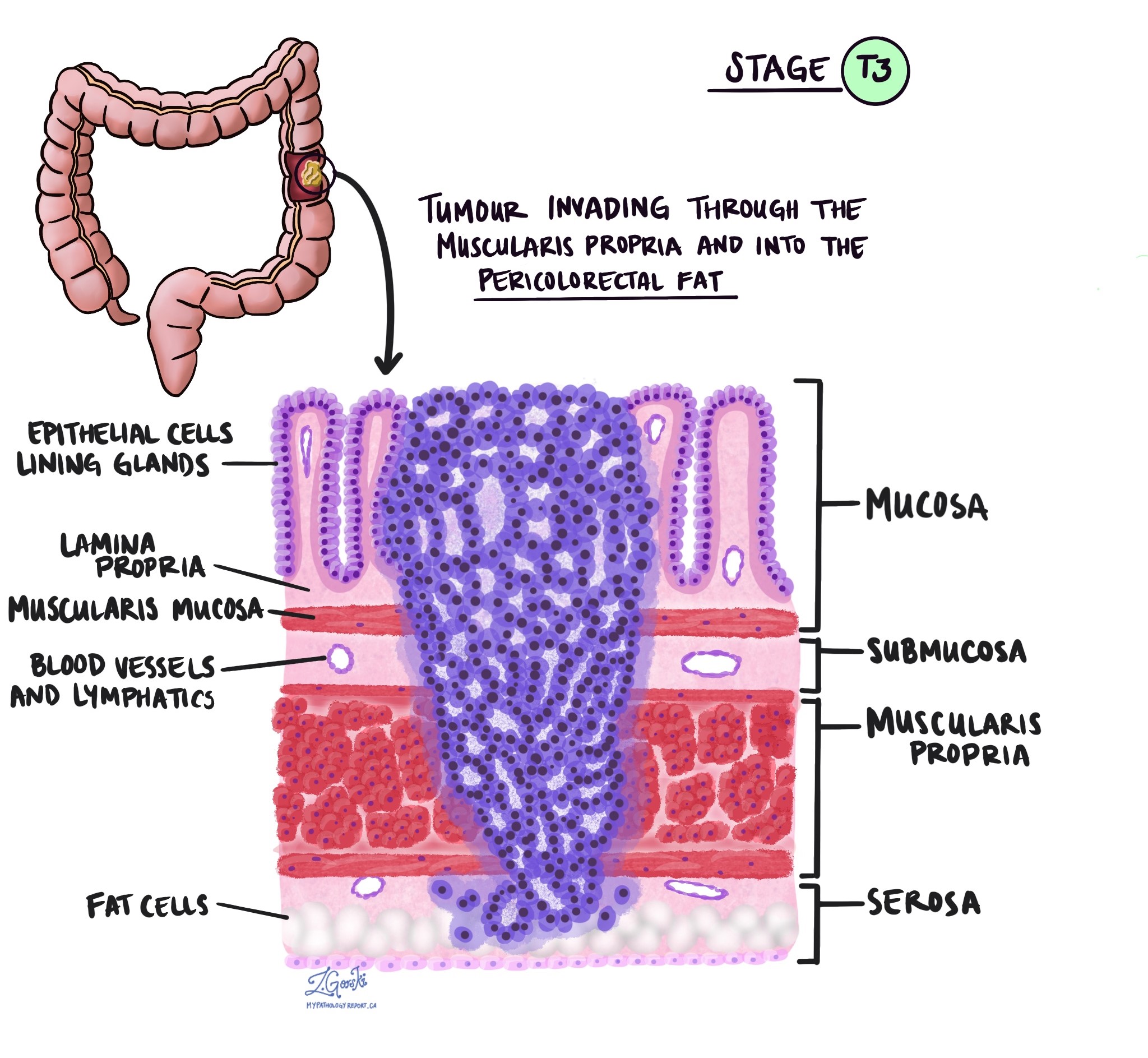
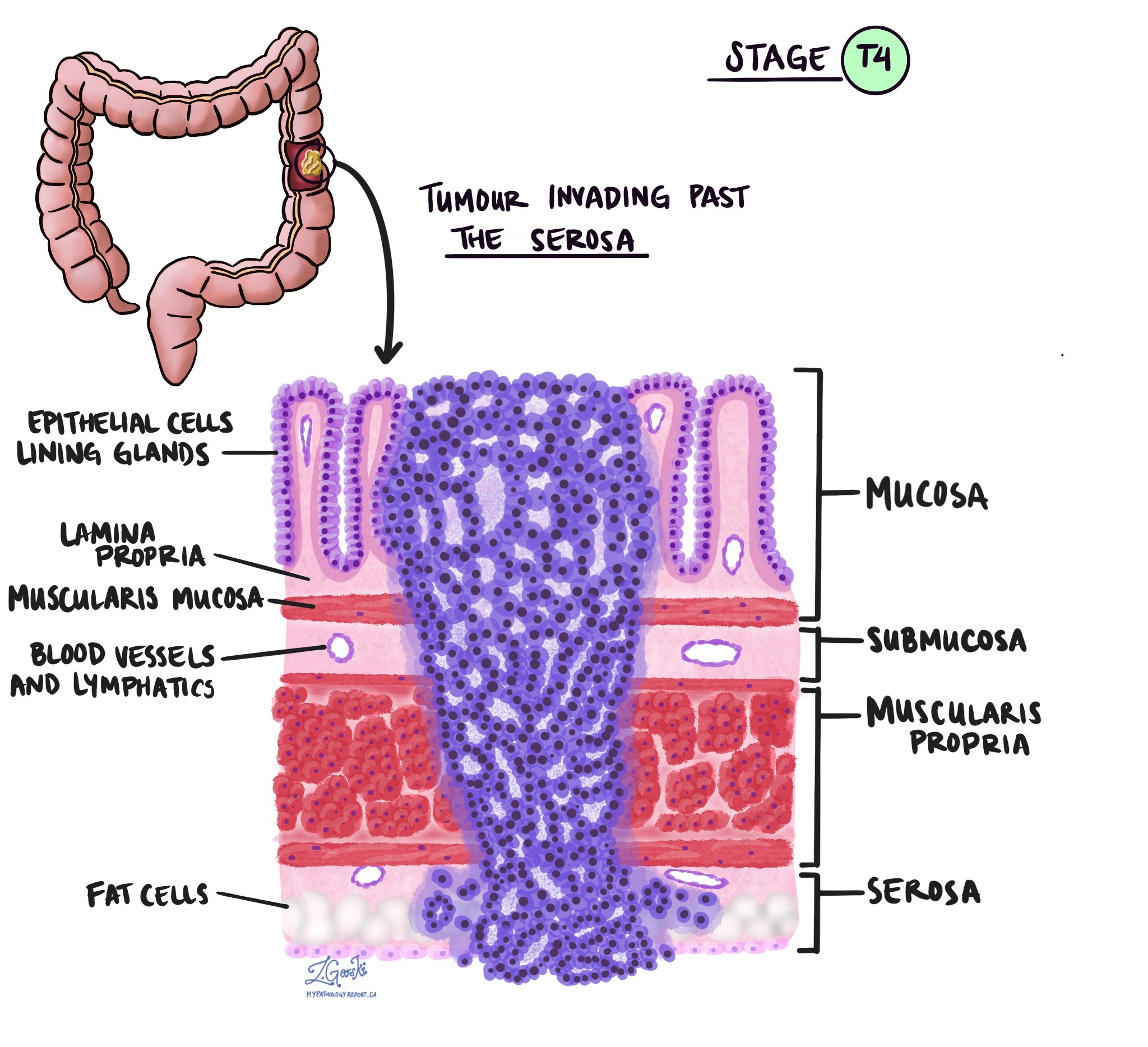
Tumor stage (pT)
The tumor stage (pT) tells us how deeply the tumor has grown into the wall of the colon or nearby tissues. The colon wall is made up of several layers, starting from the inner surface and moving outward:
-
Mucosa – The inner lining where the cancer starts.
-
Submucosa – A layer of tissue under the mucosa.
-
Muscularis propria – A thick muscle layer that helps move stool.
-
Subserosa and surrounding fat – A layer of fat around the outside of the colon.
-
Serosa – The outermost surface (not present everywhere in the colon).
pT categories
- pT1: The cancer has grown into the submucosa, which is the layer just beneath the inner lining.
-
pT2: The cancer has grown into the muscularis propria, the thick muscle layer of the colon.
-
pT3: The cancer has gone all the way through the muscle layer and into the fat and tissue surrounding the colon.
-
pT4a: The cancer has reached the outer surface of the colon (called the serosa) or has caused a perforation through the colon wall.
-
pT4b: The cancer has directly invaded nearby organs or structures, such as the bladder, uterus, or abdominal wall.
Nodal stage (pN)
The nodal stage (pN) for mucinous adenocarcinoma of the colon tells us whether the cancer has spread to nearby lymph nodes, which are small immune structures that help the body filter and fight infections. Cancer often spreads to these nodes first.
pN categories
-
pN0: No cancer is found in any of the lymph nodes that were examined.
-
pN1: Cancer is found in 1 to 3 lymph nodes, or there are tumor deposits in surrounding tissues, even if all the lymph nodes are negative.
-
pN1a: One lymph node has cancer.
-
pN1b: Two or three lymph nodes have cancer.
-
pN1c: No lymph nodes have cancer, but tumor deposits are found in the fat or tissue near the colon.
-
-
pN2: Cancer is found in 4 or more lymph nodes.
-
pN2a: Four to six lymph nodes are involved.
-
pN2b: Seven or more lymph nodes are involved.
-
If no lymph nodes were sent to the lab or could not be evaluated, the nodal stage may be listed as pNX (not assessable).
Why is staging important?
The pathologic stage helps your doctor understand how advanced the cancer is and what kind of treatment may be most effective. In general:
-
Lower stages (like pT1 or pN0) suggest an earlier cancer with a better chance of cure.
-
Higher stages (like pT4 or pN2) suggest a more advanced cancer with a higher risk of recurrence.
This information, combined with other features such as tumor grade, lymphovascular invasion, and molecular markers, helps guide decisions about chemotherapy and follow-up care.
Questions for your doctor
- How far had the tumour invaded the colon wall?
-
Were any lymph nodes involved?
-
What was the tumour grade?
-
Were there any signs of tumour deposits or tumour budding?
- Was my tumour mismatch repair proficient or deficient?
- Will I need any additional treatment such as chemotherapy?
-
What follow-up tests or procedures will I need?




