by Jason Wasserman MD PhD FRCPC
August 22, 2025
High grade serous carcinoma is the most common type of ovarian cancer. It develops from epithelial cells, which are the cells that normally cover the outside surface of the ovaries. The word serous describes the appearance of the tumor cells under the microscope, which resemble cells that line the fallopian tubes. The term high grade means that the tumor cells look very abnormal and grow quickly.
This is considered an aggressive type of ovarian cancer because it often spreads outside the ovary by the time it is diagnosed. However, it is also one of the most responsive ovarian cancers to chemotherapy.
What are the symptoms of high grade serous carcinoma?
The symptoms of high grade serous carcinoma are often vague, which can make early diagnosis difficult. Many patients experience abdominal bloating or swelling, pain or pressure in the pelvis or lower abdomen, changes in bowel or bladder habits, loss of appetite, unexplained weight loss, or fatigue. Because these symptoms are common to many conditions, ovarian cancer is often diagnosed at an advanced stage.
Who gets high grade serous carcinoma?
High grade serous carcinoma usually affects women over the age of 60, although it can occur in younger patients. It accounts for about 70% of all ovarian cancers. Risk factors include older age, a family history of breast or ovarian cancer, and inherited genetic changes such as mutations in BRCA1 or BRCA2. Protective factors include having children, breastfeeding, use of oral contraceptives, and early menopause.
What causes high grade serous carcinoma?
Most cases are caused by genetic changes in the cells of the fallopian tubes, particularly in a gene called TP53, which helps control how cells grow and divide. Almost every case has a TP53 mutation.
Some patients inherit genetic changes that increase their risk. For example, about 15% of cases are due to inherited mutations in BRCA1 or BRCA2. Rarely, mutations in other genes such as RAD51C, RAD51D, and BRIP1 also play a role. These mutations prevent the cell from repairing DNA damage, which increases the chance of developing cancer.
How is this diagnosis made?
The diagnosis of high grade serous carcinoma is usually made after a sample of tissue is removed during surgery or biopsy and examined under the microscope by a pathologist. Imaging tests such as ultrasound, CT, or MRI can help detect the tumor, but only tissue examination provides a definite diagnosis.
Blood tests may also be performed. A protein called CA125 is elevated in more than 90% of patients, although this test is not specific and can be abnormal in many noncancerous conditions.
What does high grade serous carcinoma look like under the microscope?
When examined under the microscope, high grade serous carcinoma is made up of large, abnormal cells that divide rapidly. The tumor often grows in papillary (finger-like) or solid (sheet-like) patterns. The cells have large and irregular nuclei, and the nuclei vary widely in size and shape. Tumor necrosis, which means areas of dead cancer cells, is also commonly seen.
These features help distinguish high grade serous carcinoma from less aggressive ovarian tumors.
What additional tests may be performed to confirm the diagnosis?
Pathologists often perform immunohistochemistry (IHC) to help confirm the diagnosis of high grade serous carcinoma and to rule out other types of ovarian tumors. IHC is a special test that uses antibodies and dyes to look for proteins produced by the tumor cells. The pattern of proteins can provide important clues about the origin and type of cancer.
The typical IHC findings in high grade serous carcinoma are:
-
WT1: This protein is positive in most cases of high grade serous carcinoma. A positive result helps confirm the diagnosis and supports the idea that the tumor came from tissue related to the fallopian tube.
-
p53: This protein shows abnormal staining in almost every case. The staining may be very strong in nearly all cells, completely absent, or rarely show an unusual pattern. Abnormal p53 staining reflects changes in the TP53 gene, which is a hallmark of this type of cancer.
-
PAX8: This marker is usually positive and indicates that the tumor comes from gynecologic tissue such as the ovary, fallopian tube, or uterus.
-
CA125: This protein is often positive in high grade serous carcinoma. It may also be measured in the blood as a tumor marker, although elevated blood levels can occur in other conditions as well.
-
FOLR1 (folate receptor alpha): This protein is frequently expressed in high grade serous carcinoma. Its detection is important because some newer treatments specifically target tumors that produce folate receptor alpha.
-
p16: This marker is often strongly positive in high grade serous carcinoma. While not specific on its own, it supports the diagnosis when combined with the other findings.
These results, taken together, give pathologists confidence in making the diagnosis and may also help guide treatment decisions, especially when targeted therapies are being considered.
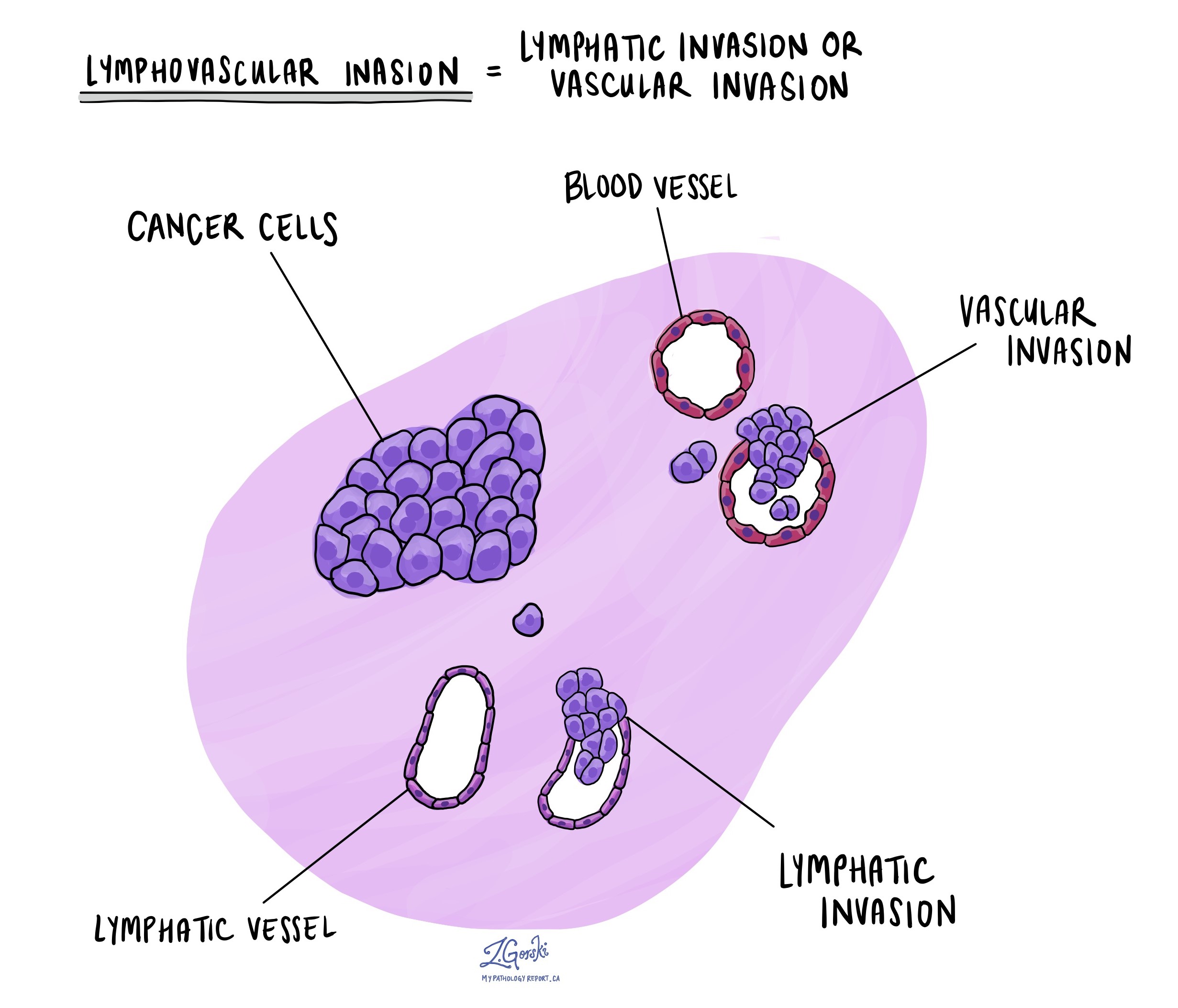
What does lymphovascular invasion mean?
Lymphovascular invasion means that cancer cells are found inside blood vessels or lymphatic channels near the tumor. Blood vessels carry blood, and lymphatic channels carry a clear fluid called lymph to nearby lymph nodes. The presence of lymphovascular invasion is important because it increases the risk that cancer cells have already spread to lymph nodes or other parts of the body.
Were tumour cells seen on the surface of the ovary or fallopian tube?
High grade serous carcinoma can spread from its original site to the surface of nearby organs such as the ovary or fallopian tube. If tumour cells are seen on the surface, this means that the cancer has already moved outside the tissue where it started. The movement of cancer cells from one site to another is called metastasis. This finding is important because once the tumour has spread beyond its original site, it is assigned a higher tumour (T) stage. A higher stage is associated with a greater chance of the cancer coming back and usually means that additional treatment will be required.
Why is it important if the tumour was received intact or ruptured?
All ovarian tumours are examined to see whether the outer surface of the ovary, called the capsule, is intact or ruptured. If the capsule is intact, it means that the tumour was contained within the ovary. If the capsule is ruptured, tumour cells may have spilled into the abdominal cavity. Rupture can happen naturally inside the body or during surgery. When the capsule ruptures inside the body, there is a higher risk of tumour spread. For this reason, rupture of the capsule changes the tumour stage and is linked to a worse prognosis. If the ovary or tumour is received in many pieces, it may not be possible for the pathologist to tell whether the rupture happened before or during surgery.
Has the tumour spread to other organs or tissues in the pelvis or abdomen?
Small tissue samples, called biopsies, are often taken from areas inside the pelvis or abdomen to look for tumour spread. These biopsies commonly come from the lining of the abdomen and pelvis, called the peritoneum. Another common site for spread is an organ called the omentum, a sheet of fatty tissue inside the abdomen that often traps tumour cells. The omentum is often removed entirely and carefully examined under the microscope.
Less commonly, tumour cells can spread to other organs such as the bladder, small intestine, or large intestine. These organs are only removed if they are directly attached to the tumour or if spread is seen during surgery. The presence of cancer cells in any of these additional sites is used to determine the tumour stage and whether there is distant metastatic disease.
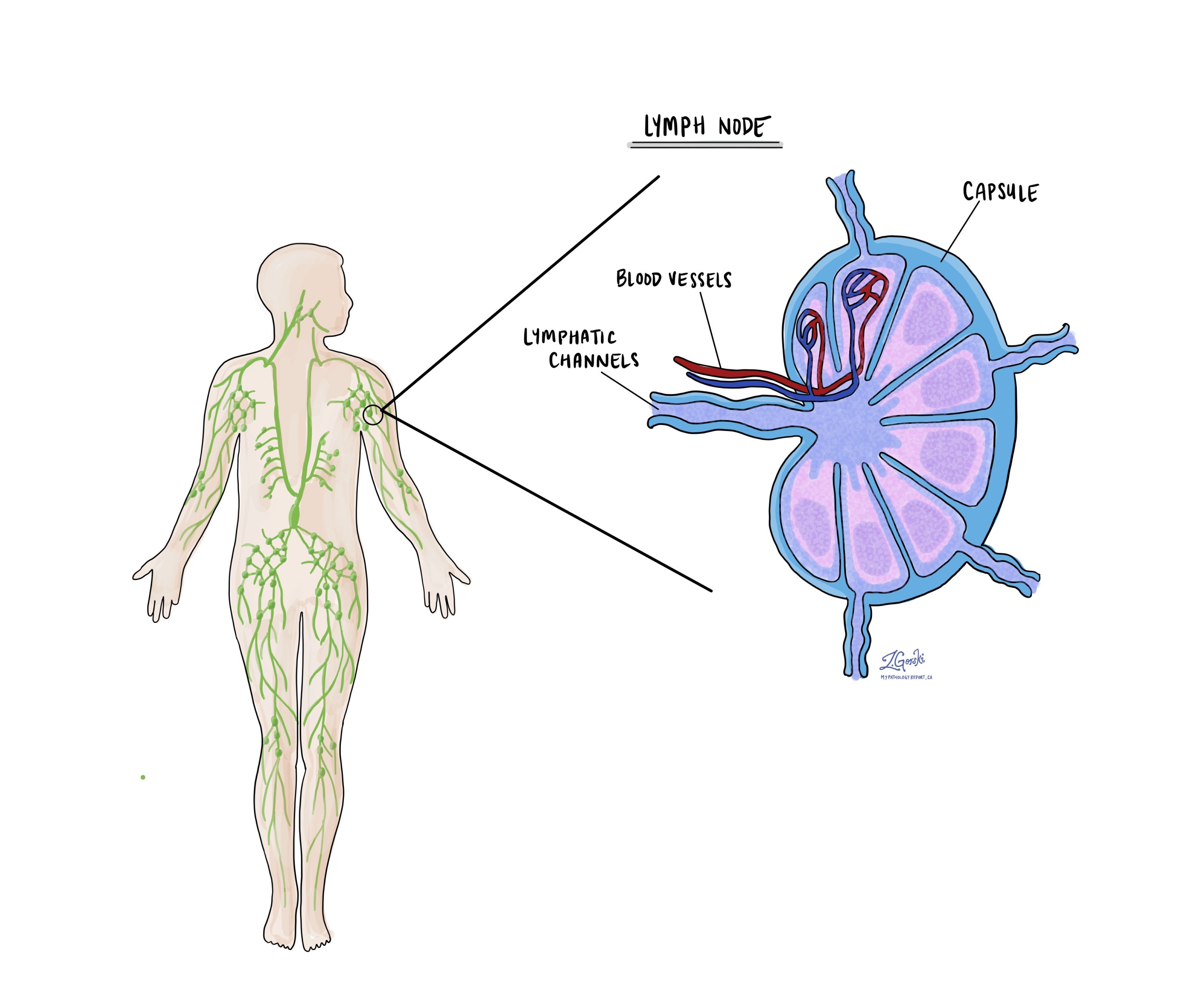
Were lymph nodes examined and did any contain cancer cells?
Lymph nodes are small immune organs located throughout the body. Cancer cells can travel to lymph nodes through lymphatic vessels. For this reason, lymph nodes are often removed and examined.
Your pathology report will include the total number of lymph nodes examined, the number that contained cancer cells, and the size of the largest group of cancer cells. This information is used to determine the nodal stage (pN). Finding cancer cells in lymph nodes increases the risk of the cancer spreading further and helps guide decisions about additional treatment.
-
Positive lymph node: A lymph node that contains cancer cells.
-
Negative lymph node: A lymph node that does not contain cancer cells.
-
Isolated tumour cells (ITCs): Very small groups of cells measuring 0.2 mm or less. These are not counted as positive when determining nodal stage.
-
Micrometastasis: A small group of cancer cells between 0.2 mm and 2 mm in size.
-
Macrometastasis: A larger group of cancer cells more than 2 mm in size.
-
Extranodal extension: Cancer cells breaking through the capsule of the lymph node into surrounding tissue. This is associated with a higher risk of the tumour growing back and may be a reason to consider additional treatment.
How is high grade serous carcinoma staged?
This cancer is staged using the FIGO and TNM systems.
-
Stage I means the cancer is limited to the ovary.
-
Stage II means it has spread to the pelvis.
-
Stage III means it has spread to the lining of the abdomen or lymph nodes.
-
Stage IV means it has spread to distant organs such as the liver or lungs.
Most patients are diagnosed at stage III or IV, when the disease has already spread outside the ovary.
What is the prognosis for high grade serous carcinoma?
The outlook depends on the stage, how much tumor can be removed during surgery, and how well the cancer responds to chemotherapy.
Most patients initially respond well to platinum-based chemotherapy, but the cancer often comes back. If the cancer returns more than six months after treatment, it is called platinum-sensitive, which has a more favorable outcome. If it returns sooner, it is called platinum-resistant, which is more difficult to treat.
Patients with inherited BRCA1 or BRCA2 mutations often respond better to treatment. New therapies called PARP inhibitors are especially effective in these cases and can be used as maintenance therapy after chemotherapy to help keep the cancer from coming back.
The five-year survival rate ranges from about 15% to 55%, depending on stage and treatment response.
Questions to ask your doctor
If you have been diagnosed with high grade serous carcinoma of the ovary, you may want to ask your doctor:
-
What stage is my cancer, and what does that mean for treatment?
-
Were any genetic changes, such as BRCA1 or BRCA2 mutations, found in my tumor?
-
Am I eligible for genetic counseling or testing for hereditary cancer syndromes?
-
Was lymphovascular invasion seen in my pathology report?
-
Were any lymph nodes involved with cancer?
-
What are my treatment options, and would I benefit from targeted therapies such as PARP inhibitors or FOLR1-directed treatments?
-
What signs of recurrence should I watch for?


