by Jason Wasserman MD PhD FRCPC and Zuzanna Gorski MD
April 19, 2025
Adenocarcinoma (“invasive adenocarcinoma”) is the most common type of colon cancer. It starts in the glandular cells that line the inside of the colon. As the tumour grows, it can invade deeper layers of the colon wall and spread to other parts of the body. The term “invasive” means that the cancer has moved beyond the inner lining of the colon into deeper tissues.
What are the symptoms of invasive adenocarcinoma of the colon?
Symptoms vary depending on the size and location of the tumour. Many people experience changes in their bowel habits, such as diarrhea, constipation, or a feeling that the bowel does not empty completely. Other symptoms include blood in the stool, abdominal pain, unexplained weight loss, and fatigue. In some cases, colon cancer may not cause noticeable symptoms until it has progressed to an advanced stage.
What causes invasive adenocarcinoma?
The exact cause of colon cancer is not always known, but several risk factors have been identified:
- Diet and lifestyle: A diet high in red and processed meats and low in fibre is linked to an increased risk of colon cancer. Lack of physical activity, obesity, and alcohol consumption are also known risk factors.
- Genetic factors: Some people inherit genetic mutations that increase their risk of developing colon cancer. Conditions like familial adenomatous polyposis (FAP) and Lynch syndrome significantly raise the likelihood of cancer.
- Chronic inflammation: Long-term inflammation in the colon, such as in Crohn’s disease or ulcerative colitis, increases the risk of developing colon cancer.
- Other risk factors: Prior radiation therapy to the pelvis, certain rare medical conditions, and smoking have been associated with an increased risk of colon cancer.
How is this diagnosis made?
The diagnosis of invasive adenocarcinoma is usually made after a colonoscopy, during which a doctor performs a biopsy to remove a small tissue sample from a suspicious area in the colon. A pathologist examines the biopsy under a microscope to confirm the presence of cancer. If cancer is found, additional tests such as imaging scans or blood tests may be performed to determine the extent of the disease.
Your pathology report for adenocarcinoma of the colon
After examining your tissue sample under the microscope, your pathologist will prepare a medical document called a pathology report. This report provides important details about your diagnosis of invasive adenocarcinoma of the colon. The type and amount of information in your pathology report will depend on whether you had a biopsy or if the tumor was completely removed during surgery.
A biopsy typically provides information about whether cancer cells are present, the type of tumor, and sometimes the grade. In contrast, a pathology report after surgery will usually contain more detailed information, including the grade of the tumor, how deeply the cancer has invaded the colon wall, the status of the surgical margins (whether the tumor was fully removed), and if cancer cells have spread to nearby lymph nodes.
Your report may also describe the results of special tests for cancer biomarkers such as mismatch repair proteins (MMR). Each of these details helps your doctor understand the extent of your disease and plan the best possible treatment. The sections below will explain each of these important topics in more detail.
Histologic grade
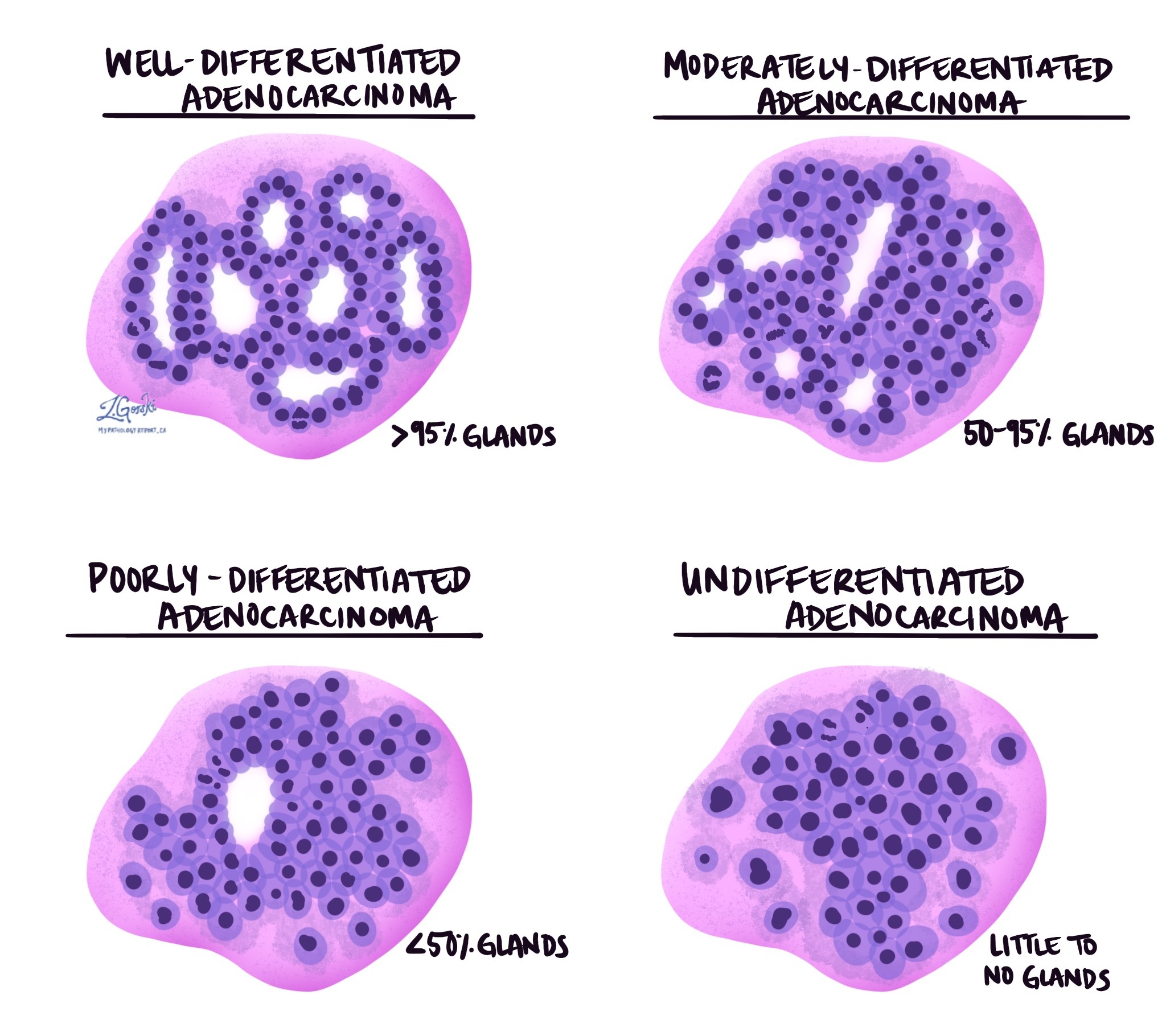
Pathologists use a grading system to describe how much the tumour cells resemble normal colon tissue. Traditionally, a four-tier system has been used based on the percentage of tumour cells forming glands.
- Well-differentiated (grade 1): More than 95% of the tumour forms glands.
- Moderately differentiated (grade 2): 50-95% of the tumour forms glands.
- Poorly differentiated (grade 3): Less than 50% of the tumour forms glands.
- Undifferentiated (grade 4): No gland formation or mucin production, with no squamous or neuroendocrine differentiation.
The most recent World Health Organization guidelines propose a simplified two-tier system:
- Low grade: This includes well- and moderately differentiated tumours (grades 1 and 2) that still form gland-like structures.
- High grade: This includes poorly differentiated and undifferentiated tumours (grades 3 and 4) with little gland formation.
Mucinous differentiation
Pathologists use the term mucinous differentiation to describe tumours that contain a large amount of extracellular mucin. Mucin is a specialised type of protein made by both normal and tumour cells. Extracellular means that the mucin was seen outside of the tumour cells. If more than 50% of the tumour is composed of mucin, the tumour is classified as a mucinous adenocarcinoma.
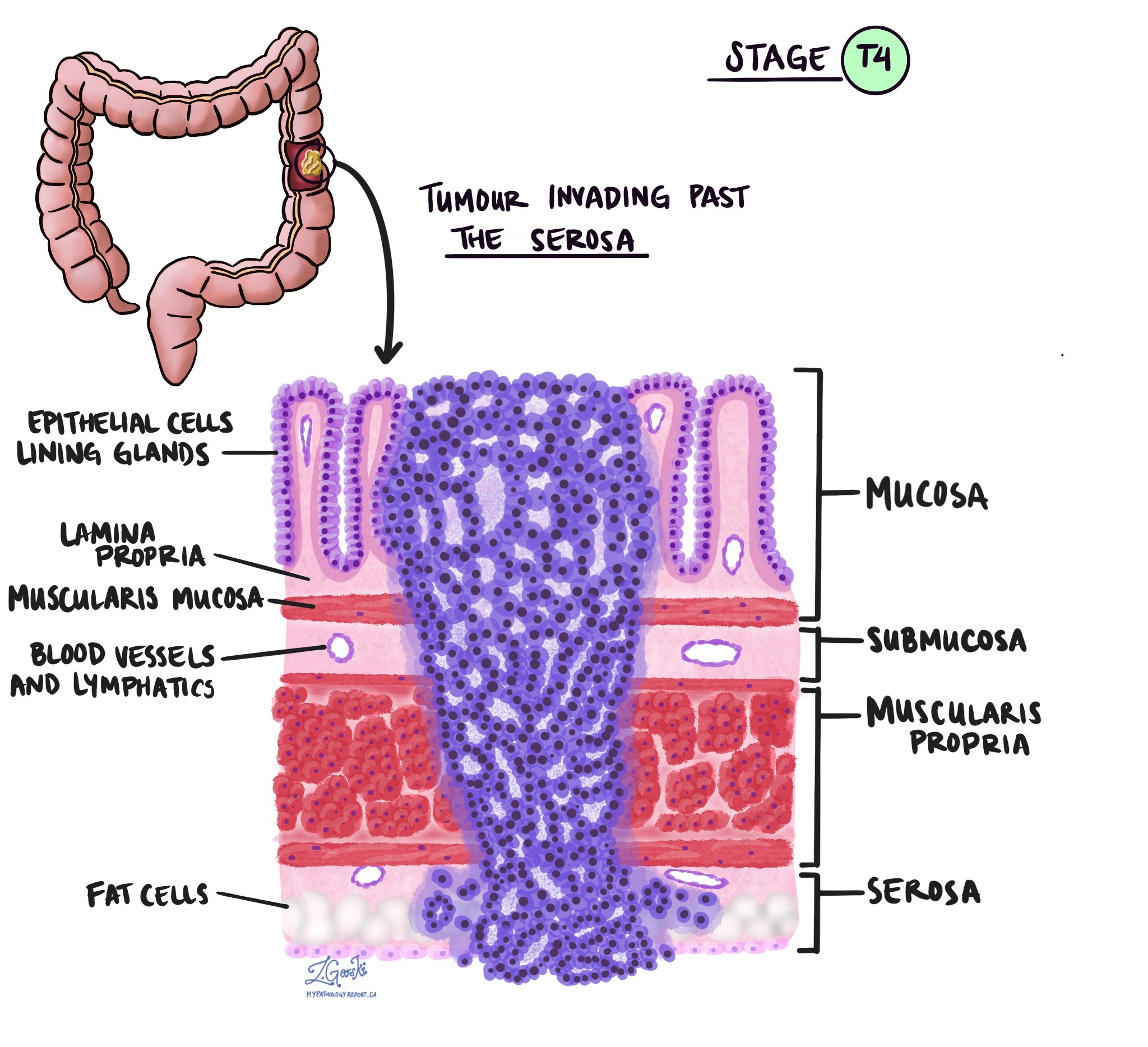
Depth of invasion and tumour stage (pT stage)
Invasive adenocarcinoma originates in the inner lining of the colon, known as the epithelium. As the tumour grows, it can extend into deeper layers:
- Lamina propria: The first layer beneath the epithelium.
- Muscularis mucosae: A thin muscle layer that separates the mucosa from deeper layers.
- Submucosa: A layer containing blood vessels and nerves.
- Muscularis propria: A thicker muscle layer responsible for moving food through the colon.
- Serosa: The outermost layer covering parts of the colon.

A tumour must invade the muscularis mucosae to be considered invasive adenocarcinoma. Tumours confined to the mucosa are called intramucosal carcinoma.
The depth of invasion is used to determine the tumour (T) stage:
- pTis: Carcinoma in situ, intramucosal carcinoma (involvement of the lamina propria with no extension through the muscularis mucosae).
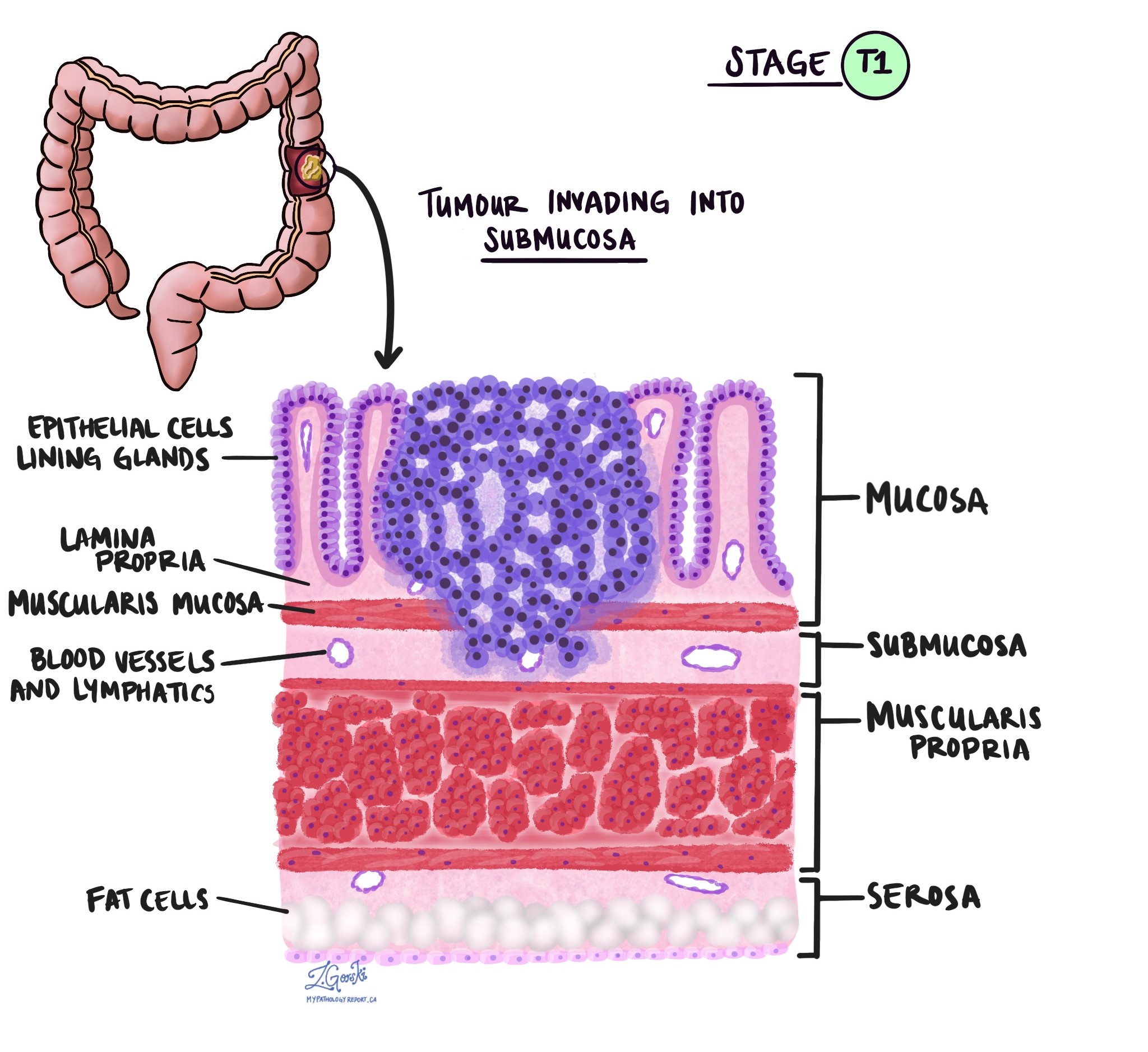
- pT1: Tumour invades the submucosa (through the muscularis mucosa but not into the muscularis propria).
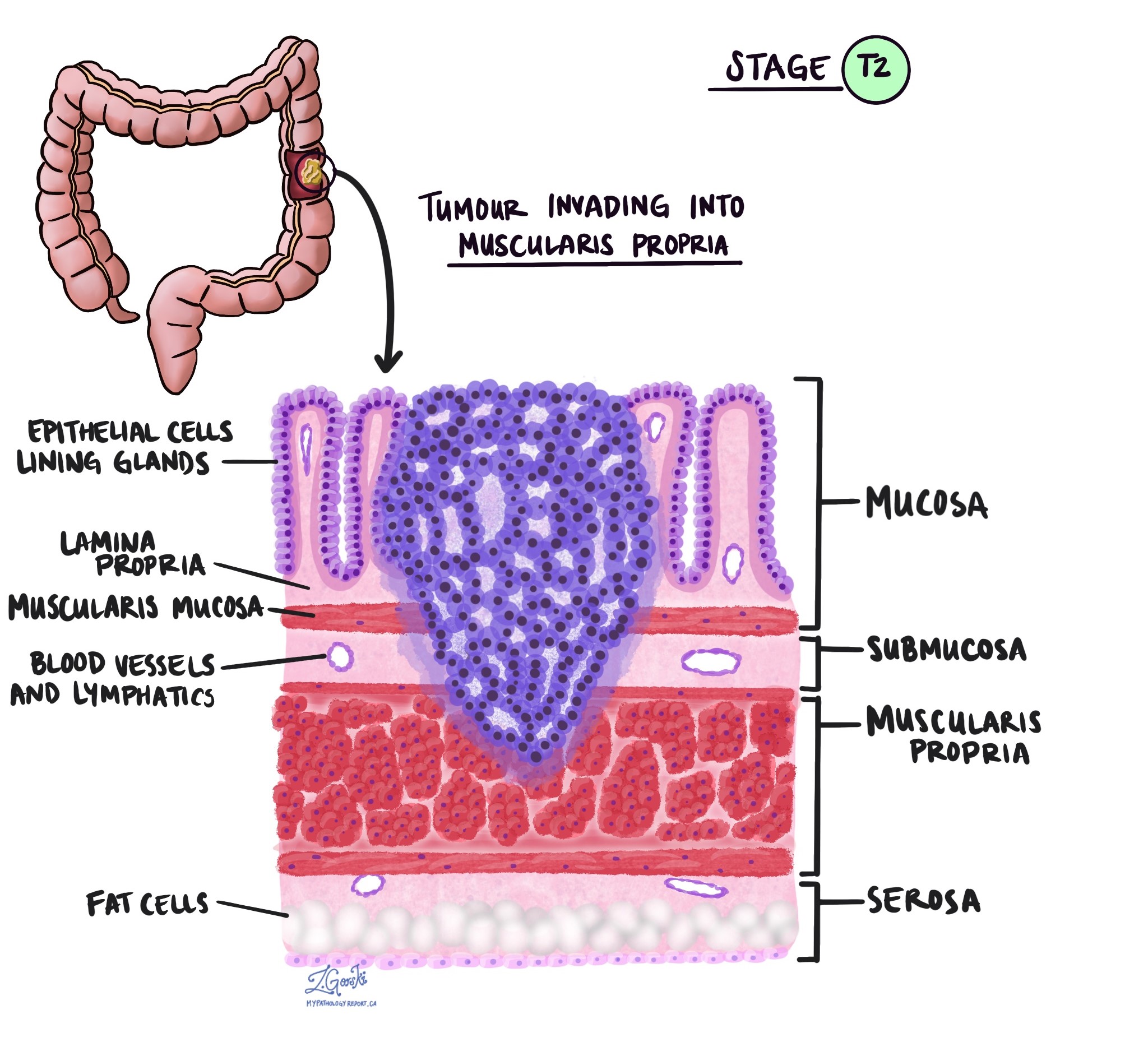
- pT2: Tumour invades the muscularis propria.
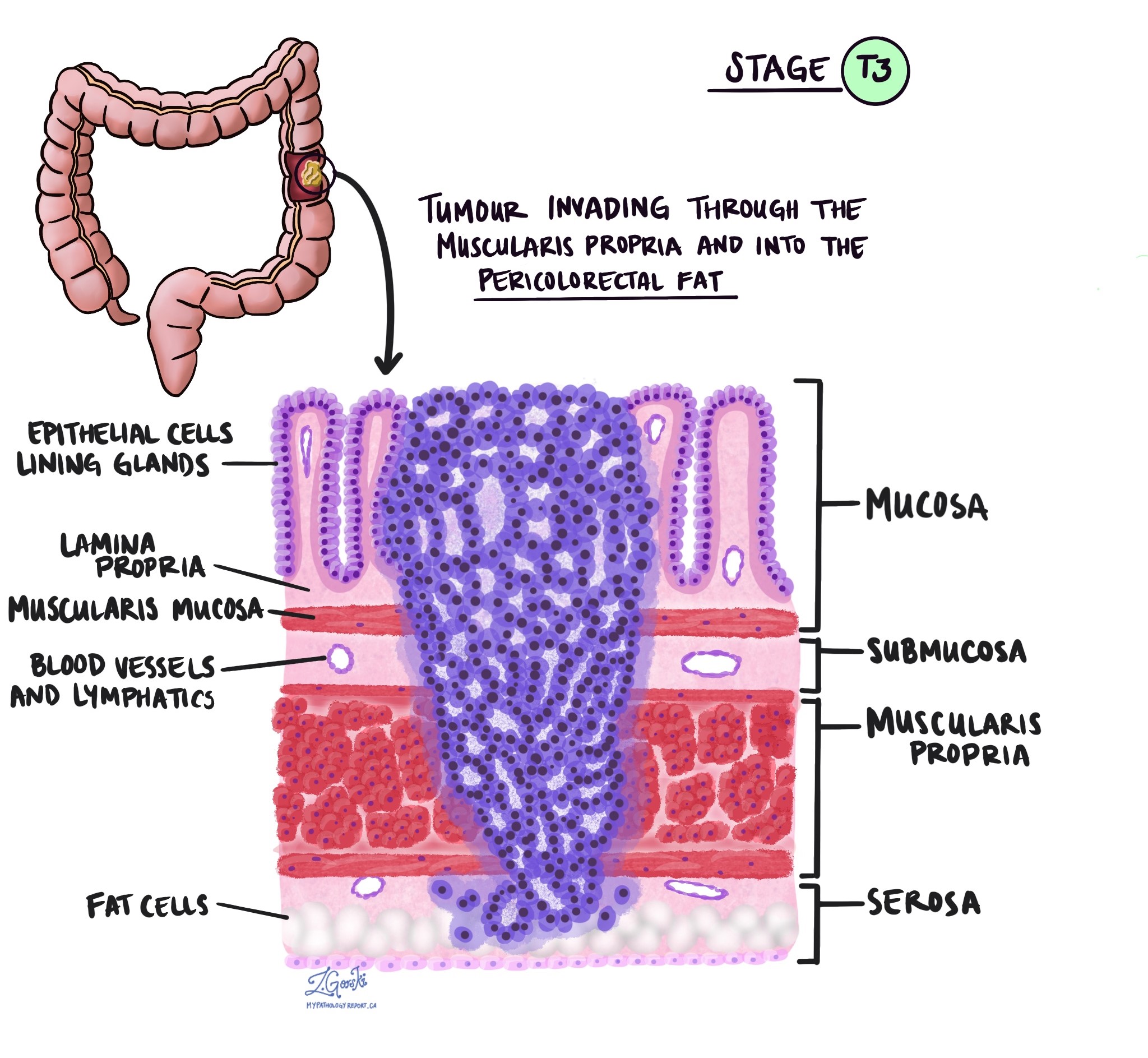
- pT3: Tumour invades through the muscularis propria into pericolorectal tissues.
- pT4: Tumour invades the visceral peritoneum or invades/adheres to an adjacent organ or structure.
- pT4a: Tumour invades through the visceral peritoneum, including gross perforation of the bowel through the tumour and continuous invasion of tumour through areas of inflammation to the surface of the visceral peritoneum.
- pT4b: Tumour directly invades or adheres to adjacent organs or structures.
Lymphovascular invasion
Lymphovascular invasion (LVI) occurs when cancer cells enter small blood vessels or lymphatic channels. This increases the risk that cancer will spread to lymph nodes or distant organs.
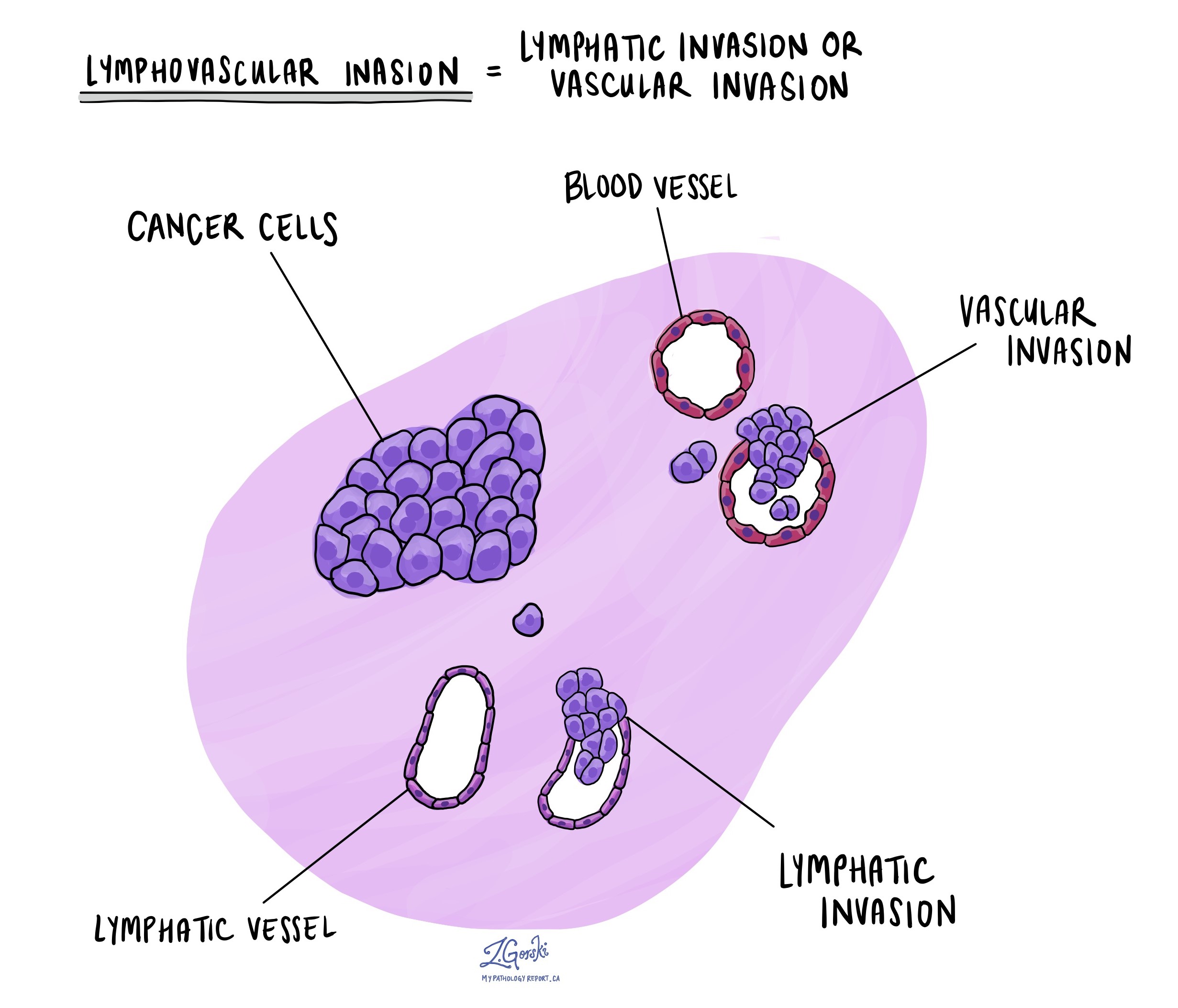
Vascular invasion
Vascular invasion means cancer cells have spread into blood vessels. It can be classified into:
- Intramural vascular invasion (IMVI): Cancer cells are found within blood vessels inside the bowel wall.
- Extramural vascular invasion (EMVI): Cancer cells have spread into blood vessels beyond the bowel wall. EMVI is considered more serious than IMVI because it is associated with a higher risk of cancer spreading to other parts of the body.
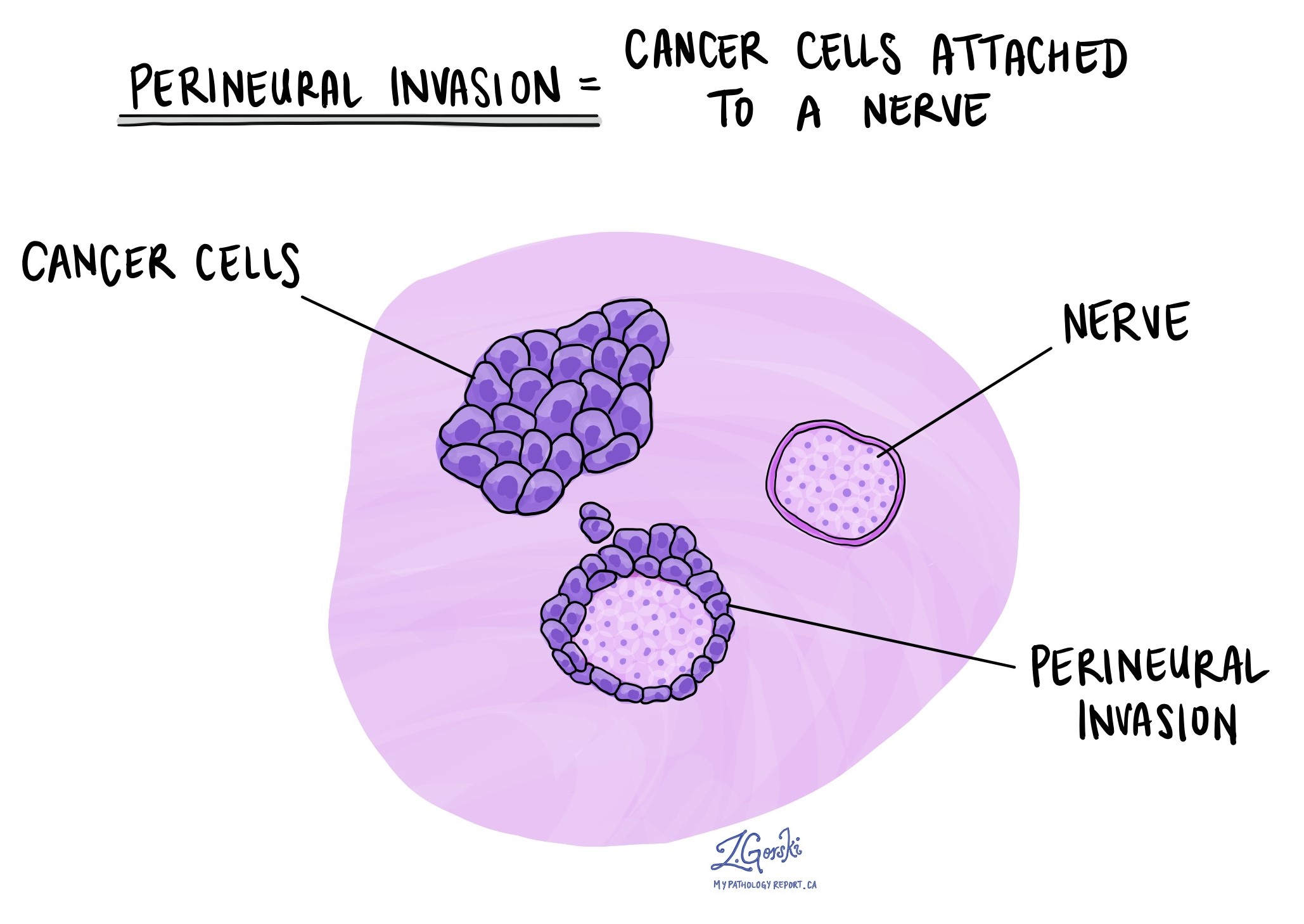
Perineural invasion
Perineural invasion (PNI) occurs when cancer cells grow along nerves. It is associated with an increased risk of local recurrence and poorer outcomes.
Tumour budding
Tumour budding refers to single cancer cells or small clusters of cells (up to four) at the edge of the tumour. Based on the number of buds seen under the microscope, tumour budding is classified as low, intermediate, or high. High grade budding is associated with more aggressive behaviour and a worse prognosis.
Margins
Margins refer to the edges of tissue removed during surgery. In colon cancer surgery, pathologists examine different types of margins:
- Proximal and distal margins: These are the ends of the colon segment removed during surgery.
- Circumferential resection margin (CRM): The outermost edge of the resected tissue, which is particularly important in rectal cancer.
- Mesocolic margin: This margin is important for tumours in the cecum. A margin is considered positive if cancer cells are found at the edge, meaning some cancer may have been left behind. A negative margin means no cancer was found at the edge, indicating the tumour was removed entirely.
Treatment effect
The tumour may shrink or disappear completely if a patient receives treatment before surgery, such as chemotherapy or radiation therapy. Pathologists assess the tumour to determine how much remains after treatment.
The response is classified as:
- No viable cancer cells (complete response, score 0).
- Single cells or rare small groups of cancer cells (near complete response, score 1).
- Residual cancer with evidence of tumour regression, but more than single cells or rare small groups (partial response, score 2).
- Extensive residual cancer with no evident tumour regression (poor or no response, score 3).
Invasive adenocarcinoma in a polyp
Invasive adenocarcinoma can develop within a colon polyp. Polyps are growths on the inner lining of the colon, and types include tubular adenoma, tubulovillous adenoma, villous adenoma, and sessile serrated lesions. When cancer is confined to the polyp and has not spread beyond it, the prognosis is generally very good, and complete removal of the polyp may be curative.
Haggit levels
In pedunculated polyps, where the polyp has a stalk, the depth of invasion is assessed using the Haggitt levels:
- Level 1: Cancer is limited to the head of the polyp.
- Level 2: Cancer invades the neck of the polyp.
- Level 3: Cancer invades the stalk of the polyp.
- Level 4: Cancer extends beyond the stalk into the submucosa of the colon wall.
Kikuchi levels
In sessile polyps, which do not have a stalk, invasion is assessed using the Kikuchi levels:
- Sm1: Superficial submucosal invasion.
- Sm2: Mid-level submucosal invasion.
- Sm3: Deep submucosal invasion.
The risk of lymph node metastasis increases with deeper levels of submucosal invasion. If cancer has invaded deeply into the submucosa, surgery to remove part of the colon may be needed to ensure complete removal of the tumour and assess for spread to lymph nodes.
Tumour deposits
Tumour deposits are small groups of cancer cells found in the fat surrounding the colon or rectum. They are located in the lymphatic drainage area of the primary tumour but do not contain identifiable lymph node tissue or blood vessels. If a tumour focus is found in a vessel, it is classified as vascular invasion rather than a tumour deposit. Similarly, if a tumour focus is found near a nerve, it is classified as perineural invasion.
Tumour deposits are important because their presence increases the risk of cancer spreading. If tumour deposits are present but no lymph nodes contain cancer, the cancer is classified as N1c, regardless of the tumour (T) stage. If positive lymph nodes are also found, the cancer is classified based on the number of involved lymph nodes (N1a or N1b), but the presence and number of tumour deposits are still noted in the pathology report. Tumour deposits are considered an adverse prognostic factor, and their presence may influence treatment decisions, such as the need for chemotherapy.
Lymph nodes
Lymph nodes are small immune organs found throughout the body. Cancer cells can spread from a tumour to these nodes via lymphatic vessels, prompting doctors to remove and examine them for cancer. This process is known as metastasis. Typically, cancer cells first migrate to lymph nodes nearest to the tumour, but more distant nodes can also be affected. Surgeons often remove the closest lymph nodes first and may take additional ones if they appear enlarged and potentially cancerous.
Pathologists examine the removed lymph nodes and report results as “positive” if cancer cells are found and “negative” if not. If cancer is detected, the report may indicate the size of the largest cluster, referred to as a “focus” or “deposit.” Extranodal extension refers to tumour cells breaking through the lymph node’s outer capsule into nearby tissue.
Examining lymph nodes is important for determining the pathologic nodal stage (pN) and gauging the risk of cancer spreading to other body parts. This information helps doctors determine whether further treatments, such as chemotherapy, radiation, or immunotherapy, are necessary.
Mismatch repair proteins
Mismatch repair proteins (MMR) are a system inside normal, healthy cells that fix mistakes in our genetic material (DNA). The system comprises different proteins, the four most common being MSH2, MSH6, MLH1, and PMS2. The four MMR proteins work in pairs to fix damaged DNA. Specifically, MSH2 works with MSH6, and MLH1 works with PMS2. If one protein is lost, the pair cannot function normally, and the risk of developing cancer increases.
Testing for mismatch repair proteins
The most common way to test for mismatch repair proteins is immunohistochemistry. This test allows pathologists to see if the tumour cells produce all four mismatch repair proteins. The results of this test are typically reported as follows:
- Normal result: Retained protein expression.
- Abnormal result: Loss of protein expression.
Mismatch repair testing is important because it can help predict how well specific treatments may work. For instance, cancers with a loss of mismatch repair protein expression are more likely to respond to immunotherapy treatments like PD-1 or PD-L1 inhibitors. This is because the many mutations often found in deficient tumours can produce new antigens that make the tumour more visible and vulnerable to the immune system.
Mismatch repair testing is also performed to identify patients who may have Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC). Lynch syndrome is a genetic disorder that increases the risk of developing various types of cancer, including esophageal cancer, colon cancer, endometrial cancer, ovarian cancer, and stomach cancer.
Cancer biomarkers for adenocarcinoma
Molecular testing may be performed on tumour tissue to look for genetic changes associated with colon cancer. These tests are typically performed using next-generation sequencing (NGS), which enables pathologists to examine multiple genes simultaneously. The specific genes tested vary by institution, but they generally assess for mutations, deletions, and rearrangements that may impact prognosis and treatment options.
Results from molecular testing are typically reported as:
- Mutated (Altered): A specific genetic change is present in the tumour.
- Wild-type (Unaltered): The gene does not contain mutations.
- Amplified or Deleted: Some genes may have extra copies (amplified) or be missing entirely (deleted), which can influence tumour behavior and response to treatment.
KRAS
KRAS is a gene that helps regulate cell growth and division. Mutations in KRAS occur in approximately 40-50% of colorectal cancers and are associated with resistance to specific targeted therapies, such as anti-EGFR monoclonal antibodies, including cetuximab and panitumumab. Tumours with KRAS mutations tend to have a more aggressive course and do not benefit from these treatments.
BRAF
BRAF mutations, particularly the V600E mutation, are found in about 8-12% of colorectal cancers. This mutation is associated with a poorer prognosis, especially in microsatellite-stable (MSS) tumours. BRAF-mutated tumours are often resistant to standard chemotherapy, but they may respond to targeted therapies that specifically inhibit the BRAF protein.
NRAS
NRAS mutations occur in about 3-5% of colorectal cancers and, like KRAS mutations, are associated with resistance to anti-EGFR therapy. NRAS-mutated tumours may have a more aggressive clinical course.
PIK3CA
PIK3CA mutations occur in about 10-20% of colorectal cancers and may be linked to resistance to some therapies, including anti-EGFR drugs. Some studies suggest that patients with PIK3CA mutations may benefit from aspirin therapy, but this remains an area of ongoing research.
Prognosis
Prognosis after a diagnosis of colonic adenocarcinoma depends on several factors, including tumour grade, depth of invasion, lymph node involvement, and presence of lymphovascular, vascular, and perineural invasion.
Molecular markers also play a role in prognosis:
- RAS mutations: About 50% of colorectal cancers have RAS gene mutations. These tumours do not respond well to specific targeted therapies.
- BRAF mutations: The BRAF p.V600E mutation is linked to a worse prognosis.
- Microsatellite instability (MSI): Tumours with high MSI tend to have a better prognosis and respond well to immunotherapy. In contrast, microsatellite-stable (MSS) tumours with BRAF mutations have a poorer prognosis.
Understanding these features helps doctors determine the best treatment options, including surgery, chemotherapy, and targeted therapies.